通过差热分析 (DTA) 研究埃洛石的热分解:机制和动力学评估
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
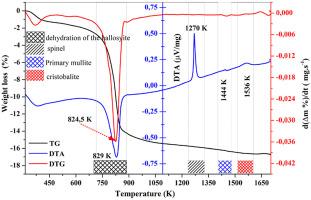
研究重点是利用差热分析(DTA)技术分析埃洛石的分解动力学。测试的温度跨度从环境温度到 1673 K,加热速率从 5 到 20 °C.min-1 不等。利用 X 射线衍射和傅立叶变换红外光谱(FT-IR)来确定在不同温度下形成的相。通过等温和非等温处理确定了埃洛石分解的活化能,其值分别约为 151.68 kJ mol-1 和 173.14 kJ mol-1。与晶体生长尺寸相关的 Ligero 方法的阿夫拉米常数参数(n)和 Matusita 方法的数值因子参数(m)都在 1.5 左右。这些研究结果表明,埃洛石的降解主要是由块状成核决定的,随后是以多面体结构为特征的元埃洛石的三维生长,并由数量一致的晶核扩散调节。埃洛石脱羟基的频率因子被确定为 8.48 × 10⁸ s-1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigation of halloysite thermal decomposition through differential thermal analysis (DTA): Mechanism and kinetics assessment
The study focused on analysing the kinetics of halloysite decomposition using the differential thermal analysis (DTA) technique. Tests were carried out across a temperature span from ambient temperature to 1673 K, employing heating rates spanning from 5 to 20 °C.min−1. X-ray diffraction and Fourier transform infrared spectroscopy (FT-IR) were utilized to identify the phases formed at different temperatures. Activation energies for halloysite decomposition were determined through isothermal and non-isothermal treatments, yielding values of approximately 151.68 kJ mol−1 and 173.14 kJ mol−1, respectively. The Ligero method's Avrami constant parameter () and the Matusita method's numerical factor parameter (), linked to crystal growth dimensions, were both around 1.5. These findings indicate that the degradation of halloysite is primarily governed by bulk nucleation, succeeded by the 3-dimensional growth of meta-halloysite characterized by polyhedron-like structure, regulated by diffusion from a consistent number of nuclei. The frequency factor for halloysite dehydroxylation was established at 8.48 × 10⁸ s⁻1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


