使用聚吡咯封装氧化锆去除维多利亚蓝染料的工艺优化:机理途径和经济评估
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
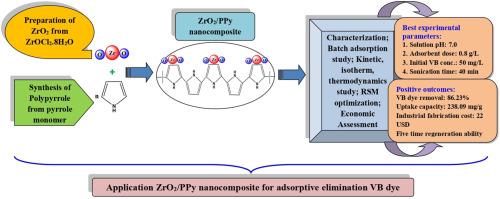
本研究开发了一种有机金属复合材料,即聚吡咯-封装氧化锆(ZrO2/PPY)纳米复合材料,并将其用于消除水体系中的维多利亚蓝染料(VB)。根据 BET 研究,ZrO2/PPY 的比表面积为 61.822 m2/g。选择溶液 pH 值为 7.0、ZrO2/PPY 纳米复合材料剂量为 0.8 g/L、超声时间为 40 分钟、初始 VB 染料浓度为 50 mg/L 作为最佳试验参数,观察到 VB 染料去除率为 86.23 (±1.15) %。VB 染料的吸附过程遵循伪二阶动力学模型和 Langmuir 等温线模型,后者得出 ZrO2/PPy 纳米复合材料的最大 VB 染料吸附容量为 238.09 mg/g。热力学研究表明,吸附研究具有自发(ΔGo< 0)和内热(ΔHo> 0)的性质。食品加工废水对 VB 染料吸附过程的阻碍最大(∼20 (±0.90) % -25 (±1.03) %),而磷酸盐(PO43-)离子对 VB 染料吸附过程的干扰最大(∼17 (±0.93) % -19 (±1.08) %)。在最佳试验参数值(吸附剂剂量:1.3 g/L,VB 染料初始浓度:20 mg/L,超声处理时间:70 min)下,VB 染料的吸附率为 0.5%:根据响应面法(RSM)的建议,VB 染料的最大去除率为 ∼96 %。静电吸引、π-π 相互作用和氢键形成是主要的吸收机制。再生研究表明,在第五次循环使用后,VB 染料消除率(%)下降了 19(±1.36)%。1.0 千克 ZrO2/PPy 纳米复合材料的实验室规模和工业规模制造成本分别为 75.74 美元和 22.20 美元。该研究结果表明,ZrO2/PPY 纳米复合材料作为一种有效且经济可行的吸附剂,具有去除废水中 VB 染料的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Process optimization of victoria blue dye removal using polypyrrole-encapsulated zirconium oxide: Mechanistic pathway and economic assessment
In this study, an organometallic composite, namely a polypyrrole-encapsulated zirconium oxide (ZrO2/PPy) nanocomposite, was developed and used to eliminate Victoria blue dye (VB) from water system. Specific surface area of the ZrO2/PPy obtained from BET study was observed to be 61.822 m2/g. Solution pH of 7.0, ZrO2/PPy nanocomposite dose of 0.8 g/L, sonication period of 40 min and initial VB dye concentration of 50 mg/L were chosen as optimal test parameters, at which 86.23 (±1.15) % of VB dye elimination was observed. The VB dye uptake process follows pseudo-second-order kinetic model and Langmuir isotherm model, with the later providing the maximum VB dye adsorption capacity of ZrO2/PPy nanocomposite as 238.09 mg/g. Thermodynamics study suggests the spontaneous (ΔGo< 0) and endothermic (ΔHo> 0) nature of the adsorption study. Food processing wastewater causes maximum hindrance (∼20 (±0.90) % −25 (±1.03) %) in the VB dye uptake process while the presence of phosphate (PO43−) ions can create highest interference (∼17 (±0.93) % −19 (±1.08) %) in the VB dye uptake process. At the optimum test parameter values (adsorbent dose: 1.3 g/L, initial VB dye concentration: 20 mg/L, sonication period: 70 min) as suggested by response surface methodology (RSM), maximum VB dye elimination of ∼96 % was observed. Electrostatic attraction, π–π interaction and hydrogen bond formation are amongst the major uptake mechanisms. Regeneration study indicates ∼19 (±1.36) % of decrease in VB dye elimination (%) after fifth cycle of reuse. The lab scale and industrial scale fabrication expense of 1.0 kg of ZrO2/PPy nanocomposite were obtained as 75.74 and 22.20 USD, respectively. The findings of this study suggest the potential of ZrO2/PPy nanocomposite as an effective and economically viable adsorbent to eliminate VB dye from wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


