基于具有可调加工性的叠氮功能化 3,4-丙烯二氧噻吩的 "点击 "单体和聚合物
IF 4.5
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
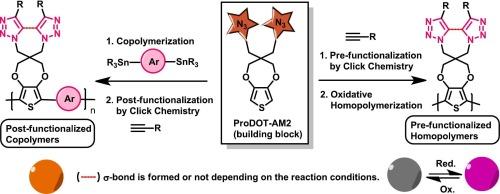
本研究介绍了 3,3-双(叠氮甲基)-3,4-二氢-2H-噻吩并[3,4-b][1,4]二氧杂环庚烷(ProDOT-AM2)作为一种多功能构筑基块单体,可通过 "点击 "1,3-二极环加成反应合成各种功能化单体和共聚物。通过与各种炔烃的点击反应,证明了这种结构单元作为多种 ProDOT 基单体前体的高效性。在催化剂存在下,ProDOT-AM2 与不同的炔烃进行 1,3-二极环加成反应,通过 "三唑-锁链 "机理生成一类新型 ProDOT 衍生物。根据所用碱的不同,可以得到两种不同类型的官能化单体,即双三唑和三唑。选定单体氧化聚合产生的聚合物在多种化学氧化还原过程中表现出较高的溶液可加工性和稳定性,显示了在化学发色方面的潜在应用。此外,一种含有 ProDOT-AM2 的共轭共聚物是通过 Stille 反应条件合成的,随后通过 "三唑-锁链 "机制与炔烃进行后官能化。通过红外和 1H NMR 进行的表征研究证实了聚合物后官能化的成功。ProDOT-AM2 的电聚合产生了一种电活性聚合物(PProDOT-AM2),这表明它在导电聚合物中具有进一步后官能化的潜在用途。总之,该方法提供了一种利用点击化学合成导电聚合物和聚合物改性的新型单体的直接方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
“Click”able monomer and polymers based on Azide-functionalized 3,4-Propylenedioxythiophene with tunable processibility
This research introduces 3,3-bis(azidomethyl)-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine (ProDOT-AM2) as a versatile building block monomer for the synthesis of variously functionalized monomers and copolymers via “click”able 1,3-dipolar cycloaddition. The efficiency of this building block as a precursor to a wide range of ProDOT-based monomers is demonstrated by click reaction with various alkynes. The 1,3-dipolar cycloaddition of ProDOT-AM2 with different alkynes in the presence of a catalyst yields a novel class of ProDOT derivatives via a “triazole-locker” mechanism. Depending on the bases utilized, two distinct types of functionalized monomers, namely bistriazole and triazole, are obtained. The resulting polymers from the oxidative polymerization of selected monomers exhibit high solution processability and stability under multiple chemical redox processes, demonstrating potential applications in chemical chromics. Additionally, a conjugated copolymer containing ProDOT-AM2 is synthesized via Stille reaction conditions and subsequently post-functionalized with alkynes via a “triazole-locker” mechanism. Characterization studies via IR and 1H NMR confirm the successful post-functionalization of the polymer. Electropolymerization of ProDOT-AM2 yields an electroactive polymer (PProDOT-AM2), indicating its potential utility in conducting polymers to be further post-functionalized. Overall, this methodology presents a straightforward approach for synthesizing a new class of monomers for conducting polymers and polymer modification using click chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


