Hic-5反义寡核苷酸抑制体内晚期肝纤维化和脂肪变性
IF 9.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
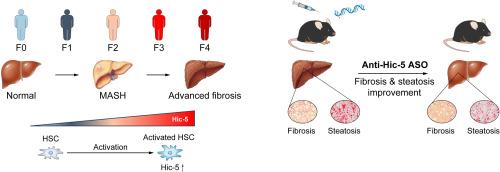
背景与ampamp; 目的慢性肝病,包括代谢功能障碍相关性脂肪性肝炎(MASH),给全球健康造成了重大负担。进行性肝纤维化可导致严重后果;然而,目前缺乏针对晚期肝纤维化的有效疗法。过氧化氢诱导的克隆-5(Hic-5)是一种局灶粘附的适配蛋白,对促进肝星状细胞的肝纤维化至关重要。本研究通过检测肝纤维化组织中肝脏Hic-5的表达,探讨其与MASH的关联,并在MASH小鼠模型中评估靶向Hic-5的反义寡核苷酸(ASOs)的治疗潜力,从而研究其临床适用性。方法对肝纤维化组织中肝脏Hic-5的表达进行病理图像分析和单细胞RNA测序。利用体外细胞模型开发并测试了靶向 Hic-5 的 ASO。结果肝脏Hic-5的表达随着纤维化的进展而增加,尤其是在晚期。单细胞 RNA 测序显示 Hic-5 主要在肝星状细胞中表达。在MASH相关纤维化中,Hic-5的表达与纤维化基因的表达相关。在MASH小鼠模型中,肝脏Hic-5的表达随着疾病的进展而增加。抗 Hic-5 ASOs 在体外能有效抑制 Hic-5 的表达,在体内能减轻晚期肝纤维化和脂肪变性,显示了其治疗潜力。抗 Hic-5 ASOs 对伴有晚期肝纤维化的 MASH 有很好的治疗效果。影响和意义:本研究调查了Hic-5在肝纤维化和脂肪性肝炎中的作用,强调了其作为治疗靶点的潜力。我们开发了一种反义寡核苷酸(ASO),这种寡核苷酸特别容易转运到肝脏,并以Hic-5为靶点。抗Hic-5 ASO对体内肝纤维化和脂肪变性具有疗效,这表明它对肝纤维化和脂肪变性具有治疗潜力。作为已获批准的核酸药物,ASO 已经取得了显著的治疗效果。因此,抗Hic-5 ASO有望成为直接用于肝纤维化和脂肪变性药物临床开发的种子化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hic-5 antisense oligonucleotide inhibits advanced hepatic fibrosis and steatosis in vivo
Background & Aims
Chronic liver diseases, including metabolic dysfunction-associated steatohepatitis (MASH), pose a significant global health burden. Progressive liver fibrosis can lead to severe outcomes; however, there is a lack of effective therapies targeting advanced fibrosis. Hydrogen peroxide-inducible clone-5 (Hic-5), an adaptor protein in focal adhesion, is critical for promoting liver fibrosis in hepatic stellate cells. This study investigated its clinical applicability by examining hepatic Hic-5 expression in human fibrotic tissues, exploring its association with MASH, and assessing the therapeutic potential of antisense oligonucleotides (ASOs) targeting Hic-5 in a MASH mouse model.
Methods
Hepatic Hic-5 expression in human fibrotic tissues underwent pathological image analysis and single-cell RNA sequencing. ASOs targeting Hic-5 were developed and tested using in vitro cell models. An in vivo MASH mouse model was used to evaluate the effects of anti-Hic-5 ASOs on advanced fibrosis and steatosis.
Results
Hepatic Hic-5 expression increased with the progression of fibrosis, particularly in advanced stages. Single-cell RNA sequencing revealed Hic-5 expression primarily in hepatic stellate cells. In MASH-associated fibrosis, Hic-5 expression correlated with the expression of fibrotic genes. In the MASH mouse model, hepatic Hic-5 expression increased with disease progression. Anti-Hic-5 ASOs effectively suppressed Hic-5 expression in vitro and attenuated advanced fibrosis and steatosis in vivo, indicating their therapeutic potential.
Conclusions
Hepatic Hic-5 expression is associated with advanced liver fibrosis and MASH. Anti-Hic-5 ASOs are promising therapeutic interventions for MASH accompanied by advanced fibrosis. These findings provide valuable insights into potential clinical treatments for advanced liver fibrosis.
Impact and implications:
This study investigated the role of Hic-5 in liver fibrosis and steatohepatitis, highlighting its potential as a therapeutic target. We developed an antisense oligonucleotide (ASO) that was particularly transportable to the liver, and targeted Hic-5. Anti-Hic-5 ASO exhibited therapeutic efficacy for liver fibrosis and steatosis in vivo, indicating its therapeutic potential for liver fibrosis and steatosis. ASOs have already achieved dramatic therapeutic effects as approved nucleic acid drugs. Thus, anti-Hic-5 ASO is expected to lead the direct generation of seed compounds for the clinical development of drugs for liver fibrosis and steatosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


