氧化还原法制备的纳米氧化锰用于去除空气中的甲苯氧化物
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
催化氧化是一种高效的挥发性有机化合物去除技术,具有巨大的潜力和发展优势。氧化消除 VOCs 的关键在于高效催化剂的开发和应用。本研究采用氧化还原法制备了纳米氧化锰催化剂,并研究了其对甲苯的催化氧化性能。煅烧温度可有效改变纳米氧化锰催化剂的表面化学成分和结构,从而有效调节催化剂表面活性中心的数量,提高纳米氧化锰催化剂对甲苯分子的吸附、活化和氧化能力。煅烧温度为 400 ℃ 时制备的以 MnO2 相为主的纳米氧化锰催化剂比表面积大、孔隙率高、活性氧丰富、氧空位多。这些结构特征有利于甲苯分子的吸附、活化和氧化,因此表现出优异的甲苯催化氧化活性。在 140 °C 的反应温度下,甲苯氧化转化率高达 99.4%,在经历了 690 分钟的稳定性测试后,甲苯转化率仍保持在 96.6% 以上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
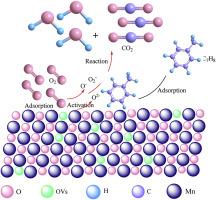
Nano-MnOx prepared by redox method for toluene oxidation removal from air
Catalytic oxidation is an efficient VOCs removal technology with great potential and development advantages. The key to the oxidative elimination of VOCs lies in the development and application of the catalyst with high efficiency. In this work, the nano-MnOx catalysts were prepared by redox method and the catalytic oxidation performance of toluene was studied. The calcination temperature could effectively change the surface chemical composition and the nano-MnOx catalyst structures, which could effectively regulate the number of active centers on the catalyst surface to improve the adsorption, activation, and oxidation ability of the nano-MnOx catalysts for toluene molecules. The nano-MnOx catalyst dominated by the MnO2 phase, which was prepared at the calcination temperature of 400 °C, had a high specific surface area, developed porosity, abundant reactive oxygen species, and oxygen vacancies. The structural characteristics are conducive to the adsorption, activation, and oxidation of toluene molecules, and thus exhibited excellent toluene catalytic oxidation activity. At the reaction temperature of 140 °C, the toluene oxidation conversion was as high as 99.4 %, and the toluene conversion remained above 96.6 % after experiencing a 690 min stability test.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


