巨噬细胞和间充质干细胞之间的相互作用塑造了矿化胶原支架的成骨和免疫调节模式
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
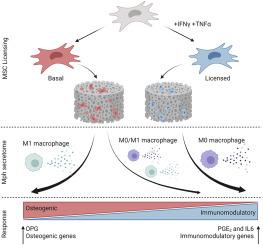
间充质干细胞(MSCs)具有高度可塑性,能够分化成一系列组织特异性基质细胞。在骨再生领域,间充质干细胞在很大程度上被认为具有成骨分化能力。间充质干细胞在受到促炎症刺激后的免疫调节潜力正日益受到重视(许可)。骨损伤后,通过巨噬细胞等常驻免疫细胞的激活,会产生促炎环境。我们介绍了使用矿化胶原支架作为仿骨体外模型,研究THP-1巨噬细胞的旁分泌与细胞间直接接触对间叶干细胞成骨和免疫调节潜力的影响。巨噬细胞的旁分泌刺激可通过上调关键转录组标记物以及产生可溶性生物大分子来增强间充质干细胞的成骨和免疫调节潜力。间充质干细胞与巨噬细胞直接共培养会降低间充质干细胞的免疫调节潜能,尤其是许可的间充质干细胞,但会增强基质重塑和巨噬细胞趋化相关基因的表达。这些数据表明,在骨再生的生物材料模型中,巨噬细胞衍生的旁分泌因子和直接接触对间叶干细胞的活性有重大影响。这项工作揭示了在临床相关性更强的细胞模型中进一步了解这些过程以指导生物材料设计的迫切需要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Crosstalk between macrophages and mesenchymal stem cells shape patterns of osteogenesis and immunomodulation in mineralized collagen scaffolds
Mesenchymal stem cells (MSCs) are highly plastic, with the capacity to differentiate into a spectrum of tissue-specific stromal cells. In the field of bone regeneration, MSCs have largely been considered for their osteogenic differentiation capacity. MSCs are increasingly being appreciated for their immunomodulatory potential following exposure to pro-inflammatory stimuli (licensing). Pro-inflammatory environments arise following bone injury via activation of resident immune cells like macrophages. We describe the use of a mineralized collagen scaffold as a bone-mimetic in vitro model to study the influence of paracrine versus direct cell-to-cell contact of THP-1 macrophages on MSC osteogenic and immunomodulatory potential. Paracrine stimuli from macrophages enhance MSC osteogenic and immunomodulatory potential via upregulation of key transcriptomic markers as well as via soluble biomolecule production. Direct co-culture of MSCs and macrophages decreased immunomodulatory potential in MSCs, especially for licensed MSCs, but enhanced matrix remodeling and expression of genes related to macrophage chemotaxis. These data demonstrate the significant effect macrophage-derived paracrine factors and direct contact have on MSC activity in a biomaterial model of bone regeneration. This work illuminates a critical need to further understand these processes in more clinically relevant cell models to inform biomaterial design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


