在水中从烯丙基醚中区域选择性合成功能化环戊-2-烯酮衍生碳酰脲和烯氨羟基巴比妥酸酯
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
一种涉及环状仲胺、活化炔烃和阿洛糖衍生物在水中的绿色一锅反应已经建立,为高产率生产(烯氨基)羟基巴比妥酸盐和 3-氨基-5-羟基环戊-2-烯酮衍生羰基脲提供了一条经济有效的选择性路线。该方法利用现成的起始材料,在温和的条件下通过三组分级联反应制备出用于药物化学和有机合成的有价值化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
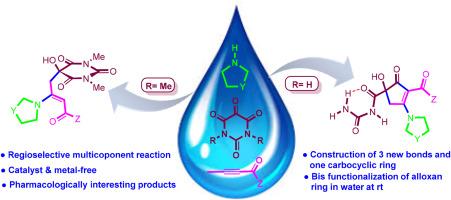
Regioselective synthesis of functionalized cyclopent-2-enone-derived carbonylureas and enaminohydroxybarbiturates from alloxans in water
A green one-pot reaction involving cyclic secondary amines, activated alkynes, and alloxan derivatives in water has been established, offering a cost-effective and selective route to (enamino)hydroxybarbiturates and 3-amino-5-hydroxycyclopent-2-enone-derived carbonylureas with high yields. This method utilizes readily available starting materials to produce valuable compounds for medicinal chemistry and organic synthesis through a three-component cascade reaction under mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


