Pd(OAc)2/CuI 催化的通过 Sonogashira 偶联的 β-内酰胺衍生物的协同炔化反应
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文开发了一种新方法,即通过串联 C-C 键活化/索诺伽希拉型交叉偶联反应,在双金属 Pd(OAc)2/CuI 催化下将 3-氯氮杂环丁烷-2-酮(β-内酰胺衍生物)与末端炔烃进行炔化。该合成包括一系列惰性 Csp3-X/Csp-H 功能化过程,涉及 3-氯氮杂环丁烷-2-酮邻接的 C(sp3)与末端炔烃的 C(sp)的耦合。最终的类似物是通过吲哚-苯并噻唑席夫碱形成、席夫碱环化生成 β-内酰胺衍生物、以及 Sonogashira 偶联生成 C(sp3)-C(sp)键来合成的。此外,吲哚、苯并噻唑和氮杂环丁烷-2-酮已被确认为具有一系列药理作用的高效杂环支架。这促使我们发现了一种具有上述每个分子的杂环化合物。两种不同金属之间的合作对于成功实施这种方法至关重要。具体来说,我们通过一条合理的机理路线,描绘了透金属化步骤中钯和铜中心之间的相互作用。通过改变催化剂和配体的负载量、碱、温度和溶剂,对反应条件进行了优化。该反应非常高效,在多种底物和官能团耐受性条件下,炔化产物的收率高达 84%,因此可作为 Sonogashira 偶联反应的功能模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
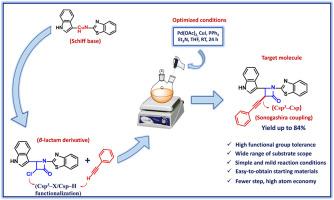
Synergistic Pd(OAc)2/CuI-catalyzed alkynylation of β-lactam derivative via Sonogashira coupling
Herein, a novel approach of bimetallic Pd(OAc)2/CuI-catalyzed alkynylation of 3-chloroazetidin-2-one (β-lactam derivative) with terminal alkyne via tandem C–C bond activation/Sonogashira-type cross-coupling reaction is developed. This synthesis encompasses a sequence of inert Csp3–X/Csp–H functionalization processes involving the coupling of the C(sp3) adjacent to the 3-chloroazetidin-2-one with the C(sp) of a terminal alkyne. The final analogs are synthesized by means of the indole-benzothiazole Schiff base formation, cyclization of the Schiff base to generate the β-lactam derivative, and Sonogashira coupling to afford a C(sp3)–C(sp) bond. In addition, indole, benzothiazole, and azetidine-2-one have been identified to be highly efficient heterocyclic scaffolds with a range of pharmacological benefits. It encouraged us to discover a hybrid compound that had each of these moieties. Cooperation between two different metals is essential for the successful implementation of this approach. Specifically, the interaction between the Pd and Cu centers in the transmetallation step is depicted through a plausible mechanistic route. The reaction conditions have been optimized by varying catalyst and ligand loadings, bases, temperatures, and solvents. The reaction is highly efficient, yielding alkynylated products in up to 84 % yield with a wide range of substrates and functional group tolerance, thereby serving as a functional model for the Sonogashira coupling reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


