利用减小尺寸的二氧化碳气泡进行汽提去除乙醇:机械和热力学夹带
IF 3.8
3区 工程技术
Q3 ENERGY & FUELS
Chemical Engineering and Processing - Process Intensification
Pub Date : 2024-09-29
DOI:10.1016/j.cep.2024.110011
引用次数: 0
摘要
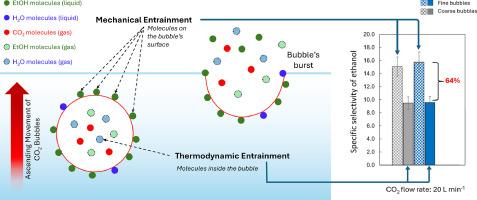
在萃取发酵过程中,机械和热力学夹带对乙醇的去除起着至关重要的作用。这些机制取决于气泡直径、乙醇浓度、液体体积、压力和温度等变量。然而,如何分别量化它们以及它们与气泡直径的关系还从未得到过研究。本研究根据工作容积为 10.0 升和 50 升的反应器中气泡的实验数据和热力学平衡分析,提出了一种评估机械夹带和热力学夹带的新方法,旨在阐明气泡直径(微泡、细泡和粗泡)在这些机制中的作用。结果表明,在特定气体流速为 0.22 至 10 vvm 的各种实验条件下,直径不超过 5 毫米的任何大小的气泡都能达到热力学平衡。同样重要的是,研究发现机械夹带可使乙醇中的气相富集 15 倍,比热力学夹带的浓度系数更高,这在最初是不可想象的。这为通过机械夹带强化挥发性化合物的汽提过程提供了一种新的可行方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ethanol removal by stripping with CO2 reduced-size bubbles: Mechanical and thermodynamic entrainments
The efficiency of stripping processes for volatile compounds is governed by mechanical and thermodynamic entrainments, which play crucial roles in ethanol removal during the extractive fermentation process. These mechanisms depend on variables such as bubble diameter, ethanol concentration, liquid volume, pressure, and temperature. However, the quantification of each of them separately and their dependence on bubble diameter have never been addressed. The present study proposes a new methodology to evaluate both mechanical and thermodynamic entrainments, based on experimental data and thermodynamic equilibrium analysis of the bubbles in reactors with working volumes of 10.0 and 50 L, aiming to clarify the role that bubble diameter (microbubbles, fine and coarse bubbles) plays in these mechanisms. The results indicated that thermodynamic equilibrium was reached for any size of bubbles up to 5 mm in diameter, under a wide range of experimental conditions with specific gas flow rates from 0.22 to 10 vvm. Equally important, mechanical entrainment was found to enrich the gas phase in ethanol by a factor of 15, representing a higher concentration factor than for thermodynamic entrainment, which was inconceivable initially. This introduces a new and promising approach to intensify the stripping process of volatile compounds by means of mechanical entrainment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
9.30%
发文量
408
审稿时长
49 days
期刊介绍:
Chemical Engineering and Processing: Process Intensification is intended for practicing researchers in industry and academia, working in the field of Process Engineering and related to the subject of Process Intensification.Articles published in the Journal demonstrate how novel discoveries, developments and theories in the field of Process Engineering and in particular Process Intensification may be used for analysis and design of innovative equipment and processing methods with substantially improved sustainability, efficiency and environmental performance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


