制备氢氧化镁的喷射空化增强水合法
IF 3.8
3区 工程技术
Q3 ENERGY & FUELS
Chemical Engineering and Processing - Process Intensification
Pub Date : 2024-10-09
DOI:10.1016/j.cep.2024.110003
引用次数: 0
摘要
在通过水合法制备氢氧化镁的过程中,原位生长和结块往往会抑制反应的进行。本研究以活性氧化镁为原料,采用射流空化技术强化水合过程。根据氢氧化镁的生长过程,分析了射流增强水合的机理。研究了反应温度(T)、反应时间(t)、固液比(s)和空化数(σ)对水合速率的影响。通过 L25(54) 正交实验探讨了各因素对水合速率影响的重要性。使用扫描电子显微镜 (SEM)、X 射线衍射 (XRD) 和比表面积分析仪对水合产物进行了表征。结果表明,影响水合速率的因素依次为空化数、反应温度、固液比和反应时间。最佳工艺参数确定为:反应温度 70 °C,反应时间 80 分钟,固液比 1:12,空化数 0.42。在这些条件下,水化率达到 94.87%,生成的片状氢氧化镁分散良好,粒度分布窄(中值粒度 D50 = 4.511 μm),BET 比表面积为 11.345 m²/g。本文章由计算机程序翻译,如有差异,请以英文原文为准。
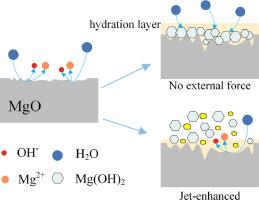
Jet cavitation-enhanced hydration method for the preparation of magnesium hydroxide
During the preparation of magnesium hydroxide via the hydration method, in-situ growth and agglomeration often inhibit the reaction. This study used active magnesium oxide as the raw material and employed jet cavitation technology to enhance the hydration process. Based on the growth process of magnesium hydroxide, the mechanism of jet-enhanced hydration was analyzed. The effects of reaction temperature (T), reaction time (t), solid-liquid ratio (s), and cavitation number (σ) on the hydration rate were investigated. An L25(54) orthogonal experiment explored the significance of each factor's impact on the hydration rate. The hydration products were characterized using scanning electron microscopy (SEM), X-ray diffraction (XRD), and a specific surface area analyzer. Results indicate that the factors affecting the hydration rate, in order of significance, are cavitation number > reaction temperature > solid-liquid ratio > reaction time. The optimal process parameters were determined to be a reaction temperature of 70 °C, reaction time of 80 min, solid-liquid ratio of 1:12, and cavitation number of 0.42. Under these conditions, the hydration rate reached 94.87 %, producing well-dispersed lamellar magnesium hydroxide with a narrow particle size distribution (median particle size D50 = 4.511 μm) and a BET specific surface area of 11.345 m²/g.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
9.30%
发文量
408
审稿时长
49 days
期刊介绍:
Chemical Engineering and Processing: Process Intensification is intended for practicing researchers in industry and academia, working in the field of Process Engineering and related to the subject of Process Intensification.Articles published in the Journal demonstrate how novel discoveries, developments and theories in the field of Process Engineering and in particular Process Intensification may be used for analysis and design of innovative equipment and processing methods with substantially improved sustainability, efficiency and environmental performance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


