非贵金属催化剂在利用 NH3 分解制氢方面的最新进展
IF 16.3
1区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
摘要
为减少全球碳足迹而向绿色、清洁和可持续能源过渡的模式推动了当前能源市场向氢经济的发展,因为氢具有非常可靠、碳中性和接近零排放的特性,但氢的安全运输和储存是一个巨大的挑战。由于氢含量高、易于储存和安全处理,NH3 是最合适的氢载体。考虑到 NH3 的能源潜力,本综述侧重于在非贵金属催化剂上热催化分解 NH3 以生产不含 COx 的 H2。考虑到成本效益和可扩展性,本综述介绍了在设计廉价催化剂方面的最新进展,这些催化剂的金属成分对全球变暖的影响较小。本文介绍了近期(尤其是 2014 年以后)Co、Ni、Fe、Mo、金属碳化物、酰亚胺、双金属和多金属催化剂与既定基准催化剂的全面调查和比较评估。本文广泛综述了各种促进剂和支撑剂对催化剂的电子特性、纹理特性、还原性和表面特性的影响。考虑到催化剂在成功进行可持续氨分解中的作用,还重点介绍了双金属相互协同作用、新型合成方法和反应机理。此外,还简要介绍了催化剂失活所面临的挑战以及应对措施。特别强调了具有在低温(450 °C)下分解氨潜力的催化剂。最后,针对进一步开发从 NH3 大规模生产 H2 的目标,介绍了总结和范围以及简要的经济评估。本文章由计算机程序翻译,如有差异,请以英文原文为准。
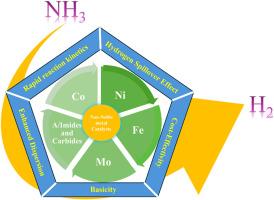
Recent advancement of non-noble metal catalysts for hydrogen production by NH3 decomposition
Paradigm transition towards green, clean and sustainable energy sources to reduce the global carbon footprint propelling the current energy market towards hydrogen economy due to the much reliable, carbon neutral and near zero emission properties though safe transportation and storage of hydrogen is a big challenge. Owing to its remarkable hydrogen content, easy storage and safe handling, NH3 is the most suitable hydrogen carrier. Considering the energy potential of NH3, this review, focus on the thermocatalytic decomposition of NH3 for COx free production of H2 on non-noble metal catalysts. Taking cost effectivity and scalability into account, recent development in design of inexpensive catalysts with metal components having less global warming potential are covered. A comprehensive survey and comparative assessment of recent (particularly post 2014) Co, Ni, Fe, Mo, metal carbides, imides, bimetallic and multimetallic catalysts with established benchmark catalysts is presented here. Effect of various promoters and supports on electronic properties, textural properties, reducibility and surface characteristics of catalyst is extensively reviewed. Mutual bimetallic synergism, novel synthetic approaches and reaction mechanism is also highlighted considering their role in successful execution of sustainable ammonia decomposition. A brief description of deactivation challenge of catalysts and measure taken to counter it is also given. Special emphasis is given to the catalysts which have potential of decomposition of ammonia at low temperature (<450 °C). Finally, summary and scope along with brief economic evaluation is presented targeting further development in large-scale production of H2 from NH3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Renewable and Sustainable Energy Reviews
工程技术-能源与燃料
CiteScore
31.20
自引率
5.70%
发文量
1055
审稿时长
62 days
期刊介绍:
The mission of Renewable and Sustainable Energy Reviews is to disseminate the most compelling and pertinent critical insights in renewable and sustainable energy, fostering collaboration among the research community, private sector, and policy and decision makers. The journal aims to exchange challenges, solutions, innovative concepts, and technologies, contributing to sustainable development, the transition to a low-carbon future, and the attainment of emissions targets outlined by the United Nations Framework Convention on Climate Change.
Renewable and Sustainable Energy Reviews publishes a diverse range of content, including review papers, original research, case studies, and analyses of new technologies, all featuring a substantial review component such as critique, comparison, or analysis. Introducing a distinctive paper type, Expert Insights, the journal presents commissioned mini-reviews authored by field leaders, addressing topics of significant interest. Case studies undergo consideration only if they showcase the work's applicability to other regions or contribute valuable insights to the broader field of renewable and sustainable energy. Notably, a bibliographic or literature review lacking critical analysis is deemed unsuitable for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


