硫醇与全氟烷基亚磺酸的全氟烷基硫代反应
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
硫醇与全氟烷基亚磺酸的全氟烷基硫代反应无需添加任何添加剂即可实现,并以良好至极佳的收率得到一系列不对称二硫化物。在减压下从反应体系中简单移除溶剂后,大多数产物都能以高纯度获得。该反应对芳基和烷基硫醇均有耐受性,并具有易于操作、无添加剂和分离过程简单等优点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
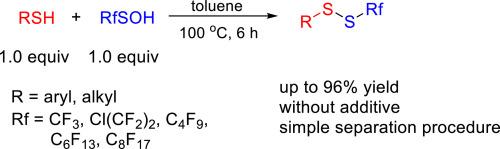
The perfluoroalkylthiolation reaction of thiols with perfluoroalkanesulfenic acids
The perfluoroalkylthiolation reactions of thiols with perfluoroalkanesulfenic acids were achieved without adding any additives, giving a series of unsymmetric disulfides in good to excellent yields. After simple removal of solvent from reaction system under reduced pressure, most products could be obtained with high purity. The reaction tolerates both aryl and alkyl thiols and has advantages such as easy-to-handle, additive-free and simple separation procedure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


