在温和条件下形成 CF3S-N 键。利用多功能试剂轻松获得三氟甲磺酰胺
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
三氟甲磺酰胺(CF3SN-分子)是一种可进一步应用的有趣产品。特别是,CF3SN 基团的存在有助于增加其亲脂性。获得此类产品的更好方法是直接对相应的胺进行三氟甲基硫代反应。本文介绍了一种在温和条件下(室温、碘催化)获得预期三氟甲烷磺酰胺后期官能化产物的有效方法。该方法基于试剂 BB23(N-对甲基苯磺酰基,N-甲基三氟甲磺酰胺)的使用,证明了该试剂在有机氟化学中的高度通用性和多价性。一些具有生物活性的化合物被成功地功能化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
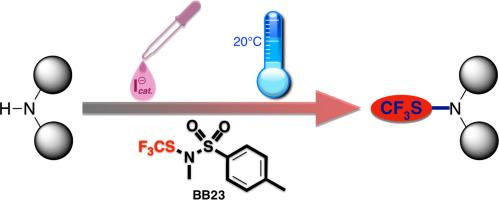
CF3S-N bond formation under mild conditions. Easy access to trifluoromethanesulfenamides with a versatile reagent
Trifluoromethanesulfenamides (CF3SN-molecules) are interesting products for further applications. In particular, the presence of the CF3SN group can contribute to increase their lipophilicity. The better way to obtain such products is the direct trifluoromethylthiolation of the corresponding amines. Herein, an efficient method under mild conditions (room temperature, iodide catalysis) is described to obtain the expected trifluoromethanesulfenamides in a late-stage functionalization. This method is based on the use of the reagent BB23 (N-tosyl, N-methyltrifluoromethanesulfenamide), demonstrating the high versatility and polyvalence of this reagent in organofluorine chemistry. Some bioactive elaborated compounds were successfully functionalized.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


