交变磁场刺激下高掺杂 Fe3O4-CA 纳米颗粒的 P(VDF-TrFE)支架上正常人成纤维细胞基因表达谱的变化
IF 5.8
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
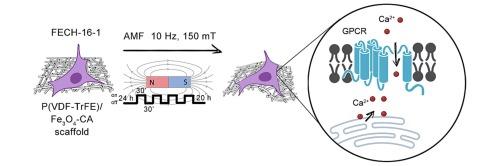
设计基于生物相容性压电聚合物和磁性纳米颗粒的新型混合磁活性支架对医学(主要用于组织再生)很有意义,因为向定居在磁活性支架上的细胞施加外部静态或交变磁场可提供远程控制细胞功能的机会。本研究介绍了电纺磁活性聚(偏氟乙烯-共三氟乙烯)[P(VDF-TrFE)]支架的制备方法,该支架高度掺杂了 20 wt% 经柠檬酸(Fe3O4-CA)修饰的磁铁矿纳米颗粒。电纺 P(VDF-TrFE)/Fe3O4-CA 支架具有无缺陷形态,纤维直径约为 1 μm,同时含有电活性 β 相(主要)和少量 γ 相。P(VDF-TrFE)纤维支架中均匀分布的高含量 Fe3O4-CA 纳米粒子使 P(VDF-TrFE)/Fe3O4-CA 复合支架的饱和磁化率高达 12.1 emu/g,并具有铁磁性。事实证明,它们与正常人体细胞具有生物相容性:正常人体成纤维细胞和人间质干细胞能粘附在支架上并保持活力。根据高通量 RNA 测序数据,成纤维细胞粘附到支架上会上调与组织再生关键阶段有关的基因,如凝血(基因 THBD 和 SERPINB2)和伤口愈合(IL24、PDGFB、F3 和 PLAU),并影响 TGFβ、BMP 和 Wnt 信号通路。磁活性 P(VDF-TrFE)/Fe3O4-CA支架表面沉积的成纤维细胞交变磁场暴露激活了细胞外和细胞内的信号通路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Changes in gene expression profile of normal human fibroblasts on P(VDF-TrFE) scaffolds highly doped with Fe3O4-CA nanoparticles under alternating magnetic field stimulation
The design of novel hybrid magnetoactive scaffolds based on biocompatible piezopolymers and magnetic nanoparticles is of interest for medicine, mainly for tissue regeneration, because application of an external either static or alternating magnetic field to cells that settled on a magnetoactive scaffold offers an opportunity for remote control of cellular functions. This study describes fabrication of electrospun magnetoactive poly(vinylidene fluoride-co-trifluoroethylene) [P(VDF-TrFE)] scaffolds highly doped with 20 wt% of magnetite nanoparticles modified with citric acid (Fe3O4-CA). The electrospun P(VDF-TrFE)/Fe3O4-CA scaffolds have defect-free morphology with a fiber diameter of approximately 1 μm and contain both an electroactive β-phase (predominantly) and a lesser amount of an γ-phase. A high content of uniformly distributed Fe3O4-CA nanoparticles within P(VDF-TrFE) fibrous scaffolds resulted in a high saturation magnetization of 12.1 emu/g and ferrimagnetic behavior of the composite P(VDF-TrFE)/Fe3O4-CA scaffolds. They were proved to be biocompatible with normal human cells: normal human fibroblasts and human mesenchymal stem cells adhered to the scaffold and retained their viability. According to high-throughput RNA-sequencing data, the adhesion of fibroblasts to the scaffolds upregulated genes related to key stages of tissue regeneration such as coagulation (genes THBD and SERPINB2) and wound healing (IL24, PDGFB, F3, and PLAU) and affected TGFβ, BMP, and Wnt signaling pathways. Alternating-magnetic-field exposure of the magnetoactive P(VDF-TrFE)/Fe3O4-CA scaffolds with fibroblasts settled on the surface activated extracellular and intracellular cell signaling pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


