瓜蒌中的一种新木质素对映体,可通过抑制 NF-κB 信号通路发挥抗神经炎症作用
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景在传统的 "瑶族 "医药体系中,瓜蒌可用于治疗风湿、风寒和炎症。以前的研究表明,从胡椒茎中提取的木质素对 LPS 刺激的小胶质细胞具有抗神经炎的潜力。目的鉴定从万寿菊茎中分离出的具有抗神经炎症活性的木质素对映体,并阐明其在体外和体内的作用机制。方法采用各种色谱和光谱方法从万寿菊茎中分离出木质素对映体并阐明其作用机制。首先通过测量一氧化氮(NO)对 LPS 刺激的 BV-2 微神经胶质细胞的抑制作用来筛选所有化合物的抗神经炎症潜力。然后用 LPS 刺激的小胶质细胞模型、小胶质细胞诱导的神经元损伤 SH-SY5Y 细胞模型和 LPS 脑室注射神经炎症小鼠模型评估了最活跃化合物的抗神经炎症功效。通过 qRT-PCR 分析、Western 印迹分析和免疫荧光染色实验进一步探讨了其潜在机制,以了解其干预途径。结果通过对 P. hancei 茎进行植物化学分析,分离出 13 对新木质素对映体(1-13),其中包括 4 对新对映体,命名为 piperhancin D-G(1-4)。成功分离出了每对对映体(1-13)的所有右手(+)和左手(-)对映体。值得注意的是,(+)-futoquinol (5)在 LPS 刺激的小胶质细胞中表现出显著的抗神经炎活性,而不像其非活性对映体(-)-5那样具有细胞毒性。代表性化合物 (+)-5 能有效抑制 LPS 刺激的 BV-2 细胞和小鼠大脑中的促炎细胞因子,并减轻 SH-SY5Y 细胞中由小胶质细胞诱导的神经元损伤。行为测试表明,(+)-5 能缓解 LPS 引起的小鼠认知功能障碍。此外,该化合物还能减轻 LPS 诱导的小鼠大脑神经元损伤和小胶质细胞活化。机理研究表明,(+)-5 阻碍了 NF-κB p65 的核转位,并下调了促炎介质,从而缓解了神经炎症。值得注意的是,(+)-futoquinol (5) 成为进一步开发治疗神经退行性疾病药物的潜在线索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
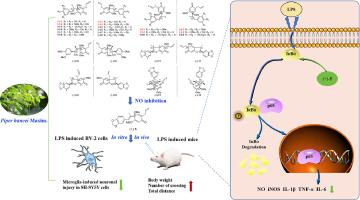
A neolignan enantiomer from Piper hancei with anti-neuroinflammatory effect by attenuating NF-κB signaling pathway
Background
In the traditional “Yao” ethno-medicine system, Piper hancei Maxim. is used to treat rheumatism, wind-cold, and inflammation. Previous studies indicate that lignans obtained from P. hancei stems have anti-neuroinflammatory potential in LPS-stimulated microglial cells. However, identification of the lignan enantiomers and the precise mechanism by which they work to reduce inflammation is yet to be explored.
Purpose
To identify the active anti-neuroinflammatory lignan enantiomers isolated from P. hancei stems and to elucidate the mechanism of action both in vitro and in vivo.
Methods
The lignan enantiomers from P. hancei stems were isolated and elucidated using various chromatographic and spectroscopic methods. The anti-neuroinflammatory potential of all the compounds was initially screened by measuring nitric oxide (NO) inhibition in LPS-stimulated BV-2 microglial cells. Then anti-neuroinflammatory efficacy of the most active compound was assessed with LPS-stimulated microglial cell model, microglia-induced neuronal injury SH-SY5Y cell model, and LPS-intracerebroventricular injection neuroinflammation mouse model. The underlying mechanism was further explored by qRT-PCR analysis, Western blot analysis, and immunofluorescence staining experiments to understand the intervention pathway.
Results
Phytochemical analysis of P. hancei stems resulted in the isolation of 13 pairs of neolignan enantiomers (1–13), including 4 new pairs named piperhancin D–G (1–4). All right-handed (+) and left-handed (–) enantiomers of each pair (1–13) were isolated successfully. Notably, (+)-futoquinol (5) demonstrated significant anti-neuroinflammatory activity without cytotoxicity, unlike its inactive enantiomer (–)-5 in LPS-stimulated microglial cells. The representative compound (+)-5 effectively suppressed pro-inflammatory cytokines in LPS stimulated BV-2 cells and mouse brains, and alleviated microglia-induced neuronal damage in SH-SY5Y cells. Behavioral tests showed that (+)-5 alleviated the LPS-induced cognitive dysfunction in mice. Furthermore, the compound was able to reduce LPS-induced neuronal damage and microglial activation in mouse brains. A mechanistic study demonstrated that (+)-5 hindered the nuclear translocation of NF-κB p65 and downregulated the pro-inflammatory mediators to relieve neuroinflammation.
Conclusion
This is the first example of both in vitro and in vivo study on the anti-neuroinflammatory effects and underlying mechanism of the neolignan enantiomers isolated from P. hancei. Notably, (+)-futoquinol (5) emerged as a potential lead for further drug development to treat neurodegenerative diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


