开发基于发光的西尼罗河病毒 MT 酶活性测量方法及其在筛选抗病毒药物中的应用
IF 4.8
Q1 MICROBIOLOGY
引用次数: 0
摘要
西尼罗河病毒(WNV)是一种黄病毒,可引起发热性疾病和严重的神经系统疾病,对全球人类健康的影响与日俱增。然而,目前仍没有适当的治疗方法来抗击 WNV 感染。因此,迫切需要开发新技术,加快发现抗这种病原体的药物。与 WNV 复制有关的主要蛋白质是非结构蛋白 5(NS5)。这种多功能蛋白含有甲基转移酶(MTase)活性,参与 RNA 5′ 端封盖的形成和病毒内部 RNA 残基的甲基化,这两种功能对于病毒过程(如 RNA 翻译和逃避先天性免疫反应)至关重要。我们已经验证了这种方法是一种灵敏且适用于鉴定 WNV MT 酶抑制剂的检测方法,并评估了广谱 MT 酶抑制剂正银霉素的抑制作用,正银霉素是通用甲基供体 S-腺苷蛋氨酸(SAM)的天然核苷类似物。通过对一小系列嘌呤衍生物的筛选,发现一种腺苷衍生物是 MT 酶活性的剂量依赖性抑制剂。该化合物的抗病毒功效在 WNV 感染中得到了进一步证实,显示出了可测量的抗病毒效果。这一结果证明了这种新方法在筛选 WNV MT 酶活性抑制剂方面的实用性,对发现和开发针对 WNV 的疗法具有特殊意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
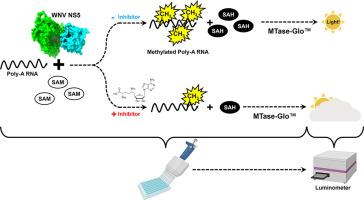
Development of a luminescence-based method for measuring West Nile Virus MTase activity and its application to screen for antivirals
West Nile virus (WNV) is a flavivirus responsible for causing febrile illness and severe neurological diseases, with an increasing impact on human health around the world. However, there is still no adequate therapeutic treatment available to struggle WNV infections. Therefore, there is an urgent need to develop new techniques to accelerate the discovery of drugs against this pathogen. The main protein implicated in the replication of WNV is the non-structural protein 5 (NS5). This multifunctional protein contains methyltransferase (MTase) activity involved in the capping formation at the 5′-end of RNA and the methylation of internal viral RNA residues, both functions being essential for viral processes, such as RNA translation and escape from the innate immune response.
We have developed a straightforward luminescence-based assay to monitor the MTase activity of the WNV NS5 protein with potential for high-throughput screening. We have validated this method as a sensitive and suitable assay for the identification of WNV MTase inhibitors assessing the inhibitory effect of the broad MTase inhibitor sinefungin, a natural nucleoside analog of the universal methyl donor S-adenosyl methionine (SAM). The screening of a small series of purine derivatives identified an adenosine derivative as a dose-dependent inhibitor of the MTase activity. The antiviral efficacy of this compound was further confirmed in WNV infections, displaying a measurable antiviral effect. This result supports the utility of this novel method for the screening of inhibitors against WNV MTase activity, which can be of special relevance to the discovery and development of therapeutics against WNV.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


