掺杂了铅、钯和铂的三种金属氮化镓晶体在吸附有害气体后的气敏响应比较
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
本文比较分析了 Pb、Pd 和 Pt 金属掺杂氮化镓(氮化镓)材料对有害气体 H2S、SO2、NH3 和 Cl2 的气体传感能力。基于密度泛函理论(DFT)计算,研究了 M-GaNNT 吸附有害气体的吸附结构、吸附能(Eads)、状态密度(DOS)、差电荷密度和前沿分子轨道。结果表明,Pb-GaNNT 的能隙在吸附 Cl2 后与吸附 Cl2 前相比变化最大,变化百分比增加了 358%,而 Pt-GaNNT 的能隙变化最小,平均值为 20.8%。金属原子的掺杂能有效提高 M-GaNNT 的气敏性,其气敏性依次为 Pb-GaNNT> Pd-GaNNT> Pt-GaNNT。通过对吸附能、灵敏响应(SR)和恢复时间(τ)的分析,预测了 M-GaNT 在气体传感器和吸附剂中的潜在应用。由于可以确定 Pb-GaNNT 对 Cl2 的吸附具有最大的 Eads(-5.883 eV)、最大的 SR(4.4 × 1015)和最大的 τ(2.9 × 1087s),因此 Pb-GaNNT 对 Cl2 气体的去除效果最好。此外,Pb-GaNNT 还是一种很好的 NH3 气体传感器,因为在 498 K 时相关的 τ 仅为 2.9 s,而且还能确定较小的 Eads(-1.232 eV)和较大的 SR(9.7 × 105)。本文的研究成果为检测和去除有害气体提供了一种新的传感器材料选择,系统的理论方法可以推广到其他系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
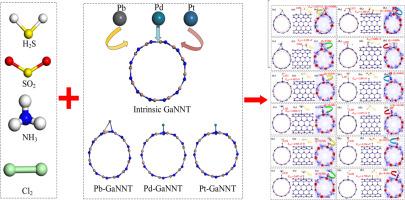
Comparison of Gas-sensitive response of three metal-doped GaNNT with Pb, Pd and Pt after adsorption of hazardous gases
In this paper, we comparatively analyze the gas-sensing ability of Pb, Pd and Pt metal-doped GaNNT (M-GaNNT) materials for the hazardous H2S, SO2, NH3 and Cl2 gases. The adsorption structure, adsorption energy (Eads), density of states (DOS), differential charge density and frontier molecular orbital of the M-GaNNT adsorbed hazardous gas have been studied based on the density functional theory (DFT) calculations. The results show that the energy gap of Pb-GaNNT performs the largest change with an increasing percentage change of 358 % after the Cl2 adsorption compared with that before the Cl2 adsorption, while the energy gap of Pt-GaNNT has the least change with an average value of 20.8 %. The doping of metal atoms can effectively improve the gas-sensitivity of M-GaNNT, and the gas-sensitivity follows the order of Pb-GaNNT> Pd-GaNNT> Pt-GaNNT. The potential applications of M-GaNNT in gas sensor and adsorbent are then predicted through the analysis of the adsorption energy, sensitive response (SR) and recovery time (τ). Pb-GaNNT performs the best Cl2 gas removal since the largest Eads (-5.883 eV), largest SR (4.4 × 1015) and largest τ (2.9 × 1087s) can be determined for Cl2 adsorption on Pb-GaNNT. Furthermore, Pb-GaNNT is a good NH3 gas sensor since the related τ is only 2.9 s at 498 K, and a small Eads (-1.232 eV) with large SR (9.7 × 105) can be determined as well. The research findings in this paper provide a new sensor material option for both the detection and the removal of harmful gases, and the systematically theoretical method can spread to other systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


