Fe1/NiSe2单原子电催化剂上的氧进化:热电级联和表面重构的作用
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
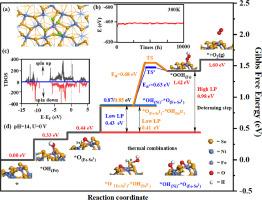
水电解中的氧进化反应(OER)是一项艰巨的挑战。在此,我们报告了二硒化镍在碱性条件下的热激活表面氧演化,特别关注了由铁单原子电催化剂支撑的 (101) 和 (100) 面。在热的辅助下,Fe1/NiSe2(101)和(100)面在 pH = 14 时表现出高效的氧进化活性。第一性原理计算和 AIMD 模拟表明,Fe1/NiSe2(101) 和 (100) 面具有出色的导电性和热稳定性,并为理解碱性条件下热-电级联 OER 过程中含氧活性物种和电催化剂之间的电子传输提供了前景广阔的思路。增强的 OER 性能取决于共吸附剂组合:Fe1/NiSe2(101)面上的*O(Fe-SeⅠ)-*OH(SeⅡ)和*O(Fe-SeⅠ)-*OH(Ni)的吸附行为导致了自激活表面重构。令人印象深刻的是,在 OER 的电位决定步骤中,关键中间产物的亲和性:Fe1/NiSe2(101)面上的*O(Fe-SeⅠ)、Fe1/NiSe2(100)面上的*O(Fe)和*OOH(Fe)通过这种自激活表面重构得到了优化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Oxygen evolution over Fe1/NiSe2 single-atom electrocatalyst: The role of thermal-electrical cascade and surface reconstruing
Oxygen evolution reaction (OER) in water electrolysis is a tough challenge. Here we report the thermally activated on-surface oxygen evolution at nickel diselenide under alkaline conditions, specifically focusing on the (101) and (100) facets supported with Fe single-atom electrocatalysts. Assisted by heat, the Fe1/NiSe2(101) and (100) facets demonstrate highly efficient activity for oxygen evolution at pH = 14. First-principles calculations and AIMD simulations illustrate excellent electrical conductivity and thermal stability of the Fe1/NiSe2(101) and (100) facets, as well as provide a promising understanding of electron transports among the oxygen-containing active species and the electrocatalysts during thermal-electrical cascade of OER under alkaline conditions. The enhanced OER performance depends on the co-adsorbate combinations: *O(Fe-SeⅠ)-*OH(SeⅡ) and *O(Fe-SeⅠ)-*OH(Ni) at the Fe1/NiSe2(101) facet, whose adsorption behaviors lead to self-activated surface reconstruing. Impressively, the affinity of the key intermediates at the potential-determining steps of OER: *O(Fe-SeⅠ) at the Fe1/NiSe2(101) facet, *O(Fe) and *OOH(Fe) at the Fe1/NiSe2(100) facet, is optimized by such self-activated surface reconstruing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


