通过密度泛函理论深入了解锂介导的氮还原反应机理
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
锂介导的电化学氮还原反应(NRR)作为哈伯-博什过程的替代方法,因其高远达效率和可重复性而受到越来越多的关注。然而,对其机理的有限了解阻碍了其催化性能的进一步提高。本研究致力于利用密度泛函理论研究锂介导的无还原反应过程及其内在机理。研究发现,Li6N2、Li7N2 原子团、Li3N (001) 和 Li3N (110) 层稳定地存在于沉积的 Li (001) 层上。建立了置换模型来描述以乙醇为质子源的氢化过程,揭示了 Li 原子与 H 原子间的置换是一个自发的热过程。基于置换机制,界面结构和反应位点的覆盖率是决定氨形成的两个主要因素。这些发现进一步加深了我们对锂介导 NRR 机理的理解,有助于合理设计锂介导 NRR 的实验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
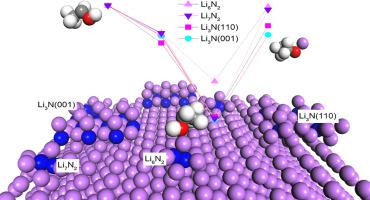
Theoretical insight into the mechanism of Li-mediated nitrogen reduction reaction by density functional theory
Lithium-mediated electrochemical nitrogen reduction reaction (NRR) as an alternative to the Haber-Bosch process has attracted increasing attention because of its high faradaic efficiency and reproducibility. However, the limited understanding of the mechanism has hampered further improvement of its catalytic performance. This work has endeavored to study the process of Li-mediated NRR and its underlying mechanism using density functional theory. It is founded that the Li6N2, Li7N2 atom groups, Li3N (001) and Li3N (110) layer stably exist on the deposited Li (001) layer. The replacing model has been established to describe the hydrogenation process with ethanol as a proton source, revealing that the replacement between Li and H atom is a spontaneously thermal process. Based on the replacing mechanism, the structure of interface and coverage rate of reactive sites are the two main factors that determine the ammonia formation. These findings further our understanding of Li-mediated NRR mechanism and will be helpful for the rational design of experiments of Li-mediated NRR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


