非致病特洛伊木马 Nissle1917 通过 PINK1/Parkin 通路触发有丝分裂,阻止结肠癌的发生
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
细菌介导的抗肿瘤疗法因其天生的肿瘤靶向能力和出色的免疫激活特性而受到广泛关注。然而,细菌疗法的临床批准仍然遥遥无期,这主要是由于平衡安全性与提高体内疗效之间的巨大挑战。在这项研究中,利用益生菌大肠杆菌 Nissle1917(EcN)治疗结肠癌是一种很有前景的方法,因为它不含毒力因子,具有高度的安全性,而且由于其必须厌氧的特性,具有靶向肿瘤的潜力。具体来说,我们描述了红细胞(RBC)膜伪装的 EcN(称为特洛伊木马 EcN@RBC),它通过降低线粒体膜电位(MMP)触发肿瘤细胞凋亡,随后激活与有丝分裂相关的 PINK1/Parkin 通路。同时,有丝分裂引起的 MMP 下降会破坏线粒体通透性转换孔(MPTP),导致细胞色素 C 的释放,进而诱导细胞凋亡。此外,通过与自噬激活剂雷帕霉素联合使用,还观察到了协同效应,增强了体内的抗肿瘤功效。这些发现提供了对益生菌介导的抗肿瘤机制的新见解,并强调了 EcN@RBC 对结肠癌患者的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
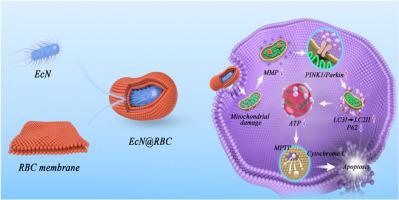
Non-pathogenic Trojan horse Nissle1917 triggers mitophagy through PINK1/Parkin pathway to discourage colon cancer
Bacteria-mediated antitumor therapy has gained widespread attention for its innate tumor-targeting capability and excellent immune activation properties. Nevertheless, the clinical approval of bacterial therapies remains elusive primarily due to the formidable challenge of balancing safety with enhancing in vivo efficacy. In this study, leveraging the probiotic Escherichia coli Nissle1917 (EcN) emerges as a promising approach for colon cancer therapy, offering a high level of safety attributed to its lack of virulence factors and its tumor-targeting potential owing to its obligate anaerobic nature. Specifically, we delineate the erythrocyte (RBC) membrane-camouflaged EcN, termed as Trojan horse EcN@RBC, which triggers apoptosis in tumor cells by mitigating mitochondrial membrane potential (MMP) and subsequently activating the PINK1/Parkin pathway associated with mitophagy. Concurrently, the decline in MMP induced by mitophagy disrupts the mitochondrial permeability transition pore (MPTP), leading to the release of Cytochrome C and subsequent apoptosis induction. Moreover, synergistic effects were observed through the combination of the autophagy activator rapamycin, bolstering the antitumor efficacy in vivo. These findings offer novel insights into probiotic-mediated antitumor mechanisms and underscore the therapeutic potential of EcN@RBC for colon cancer patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


