具有定制三维结构的混合构建体,用于引导工程组织中的预血管形成
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
结合不同结构成分的混合三维构建体为功能性组织工程提供了独特的机会。在这些构建体中创建功能性微血管网络对于促进与宿主血管的整合并确保成功移植至关重要。在这里,我们介绍了一种混合三维系统,其中聚(环氧对苯二甲酸乙二醇酯)/聚(对苯二甲酸丁二醇酯)纤维支架与果胶水凝胶相结合,以提供内部地形并引导微血管床的形成。将人类内皮细胞(EC)和间充质基质细胞(MSC)播种到该系统中的顺序/方法对微血管的形成有重大影响。将内皮细胞直接播种到纤维支架上,然后再加入水凝胶包埋的间充质干细胞,可获得最佳效果。这种方法有助于沿着纤维形成高度定向的微血管网络。这些网络具有管腔,由基底膜支撑,并由类周细胞稳定,在体外至少可持续 28 天。此外,在促血管生成和骨诱导条件下培养可诱导间充质干细胞成骨分化,而不会影响微血管的形成。在小鼠皮下植入后,预血管化的构建体被宿主血管浸润,2 周后仍存在人类微血管。总之,所提出的混合三维系统与优化的细胞播种方案相结合,提供了一种有效的方法来引导形成稳健且几何定向的微血管,使其在组织工程应用中大有可为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
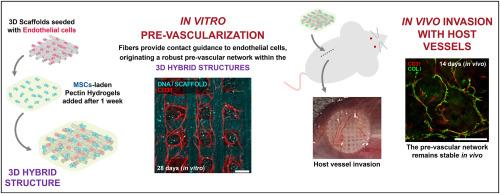
A hybrid construct with tailored 3D structure for directing pre-vascularization in engineered tissues
Hybrid 3D constructs combining different structural components afford unique opportunities to engineer functional tissues. Creating functional microvascular networks within these constructs is crucial for promoting integration with host vessels and ensuring successful engraftment. Here, we present a hybrid 3D system in which poly (ethylene oxide terephthalate)/poly (butylene terephthalate) fibrous scaffolds are combined with pectin hydrogels to provide internal topography and guide the formation of microvascular beds. The sequence/method of seeding human endothelial cells (EC) and mesenchymal stromal cells (MSC) into the system had a significant impact on microvessel formation. Optimal results were obtained when EC were directly seeded onto the fibrous scaffold, followed by the addition of hydrogel-embedded MSC. This approach facilitated the development of highly oriented microvascular networks along the fibers. These networks were lumenized, supported by a basement membrane, and stabilized by pericyte-like cells, persisting for at least 28 days in vitro. Furthermore, culture under pro-angiogenic and osteoinductive conditions induced MSC osteogenic differentiation without impairing microvessel formation. Upon subcutaneous implantation in mice, the pre-vascularized constructs were infiltrated by host vessels, and human microvessels were still present after 2 weeks. Overall, the proposed hybrid 3D system, combined with an optimized cell-seeding protocol, offers an effective approach for directing the formation of robust and geometrically oriented microvessels, making it promising for tissue engineering applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


