将外泌体静电吸附到三维制造的硅酸钙/聚己内酯上,促进骨再生
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
外泌体在骨再生中的应用备受关注,但其活性低、降解快、递送不准确等问题阻碍了其在临床应用中的使用。因此,人们需要一种外泌体整合输送平台。硅酸钙(Ca-Si)具有促进羟基磷灰石形成、成骨细胞增殖和分化的显著能力,因此被认为是最有希望用于骨再生的生物陶瓷之一。然而,Ca-Si 也有其局限性,如降解率高导致 pH 值偏高。在此,我们提出了一种骨再生平台:浸入涂有外泌体的聚己内酯(PCL)中的三维人造钙硅支架。这种设置提高了孔隙率、机械强度和天然羟基磷灰石的形成。与裸 PCL 相比,钙硅的加入增加了支架上附着的外泌体数量,并能更持久地控制外泌体的释放。将外泌体包裹的支架移植到小鼠的皮下组织时,外泌体包裹的支架表现出良好的细胞附着和成骨分化能力,显著提高了生物相容性和干细胞的原位募集能力。小鼠腓骨骨缺损动物模型证实了外泌体附着支架的骨再生功效。这些研究结果表明,外泌体包裹的 Ca-Si/PCL 支架有可能成为治疗关键骨缺损的成骨平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
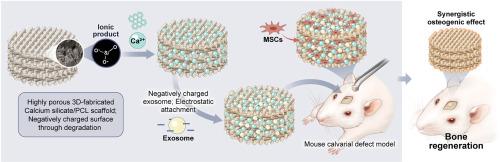
Electrostatic attachment of exosome onto a 3D-fabricated calcium silicate/polycaprolactone for enhanced bone regeneration
Exosomes have garnered attention for use in bone regeneration, but their low activity, rapid degradation, and inaccurate delivery have been obstacles to their use in clinical applications. As such, there exists a need for an exosome-integrated delivery platform. Calcium silicate (Ca-Si) is considered one of the most promising bioceramics for bone regeneration because of its remarkable ability to promote hydroxyapatite formation, osteoblast proliferation, and differentiation. However, Ca-Si has limitations, such as a high degradation rate leading to high pH values. Here, we propose a bone regeneration platform: three-dimensional-fabricated Ca-Si scaffolds immersed in polycaprolactone (PCL) coated with exosomes. This setup enhanced porosity, mechanical strength, and natural hydroxyapatite formation. Ca-Si incorporation increased the quantity of attached exosomes on the scaffold and enabled more sustainable control of their release compared to bare PCL. The exosome-coated scaffold exhibited excellent cell attachment and osteogenic differentiation, significantly increasing biocompatibility and the in situ recruitment of stem cells when transplanted into the subcutaneous tissue of mice. The bone regenerating efficacy of the exosome-attached scaffold was confirmed using a mouse calvarial bone defect animal model. These findings suggest a potential application of exosome-coated Ca-Si/PCL scaffolds as an osteogenic platform for critical bone defects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


