用于协同增强乳腺癌放射免疫疗法的癌细胞膜包被 siRNA Decorated Au/MnO2 纳米敏化剂
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
放疗在乳腺癌的临床治疗中起着至关重要的作用。然而,传统的 X 射线放疗因其肿瘤特异性低和肿瘤微环境介导的治疗耐受性而极大地限制了其疗效。在此,我们提出了一种新型的纳米放疗增敏策略,设计并构建了一种癌细胞膜包被siRNA装饰的金/二氧化锰纳米增敏剂(R&F@Au/MnO2-CM),以协同增强乳腺癌的放射免疫治疗。在这种集成纳米增感剂中,从 4T1 乳腺癌细胞中提取的癌细胞膜(CM)被用于靶向功能,而 Au/MnO2 则被设计用于改善 X 射线吸收和缓解肿瘤缺氧。此外,PD-L1 siRNA(R)用于下调肿瘤细胞中 PD-L1 的表达。在 4T1 乳腺癌原位小鼠模型中,R&F@Au/MnO2-CM 在静脉注射后通过 CM 介导的同源靶向作用准确识别了肿瘤,并通过近红外-935(F)的近红外-II 荧光成像进行了实时监测。随后,由于 Au/MnO2 在 X 射线照射下具有很强的辐射吸收特性和催化氧生成特性,实现了放疗敏感性。此外,还通过下调 PD-L1 改善了肿瘤的免疫抑制微环境,增强了协同抗肿瘤效果。我们的研究结果表明,将靶向增强放射治疗与免疫激活相结合是一种很有前景的肿瘤治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
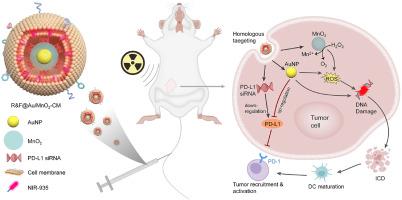
Cancer cell membrane-coated siRNA-Decorated Au/MnO2 nanosensitizers for synergistically enhanced radio-immunotherapy of breast cancer
Radiotherapy plays a critical role in the clinical treatment of breast cancer. However, the efficacy of traditional X-ray radiotherapy is greatly limited by its low tumor specificity and treatment tolerance mediated by the tumor microenvironment. Herein, we proposed a novel nano-radiotherapy sensitization strategy to design and construct a cancer cell membrane-coated siRNA-decorated Au/MnO2 nanosensitizer (R&F@Au/MnO2-CM) to synergistically enhance radio-immunotherapy for breast cancer. In the integrated nanosensitizer, the cancer cell membrane (CM) derived from 4T1 breast cancer cells is utilized for targeted functionality, while Au/MnO2 is designed to improve X-ray absorption and alleviate tumor hypoxia. Additionally, PD-L1 siRNA (R) is used to downregulate PD-L1 expression in tumor cells. In an in situ mouse model of 4T1 breast cancer, R&F@Au/MnO2-CM demonstrated accurate tumor identification via CM-mediated homologous targeting after intravenous injection, which was monitored in real-time through NIR-II fluorescence imaging of NIR-935 (F). Subsequently, the radiotherapy sensitivity was achieved due to the strong radiation absorption properties and oxygen generation through catalysis of Au/MnO2 upon X-ray irradiation. Furthermore, the immunosuppressive microenvironment of the tumor is improved by downregulating PD-L1, enhancing synergistic anti-tumor effect. Our findings demonstrate a promising approach for tumor treatment by combining targeted enhanced radiotherapy with immune activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


