利用氢氧化铝基质中夹杂的钯纳米颗粒修饰的炭黑浆电极选择性检测生物柴油样品中的 2(3)-t-Butyl-4-hydroxyanisole 抗氧化剂
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
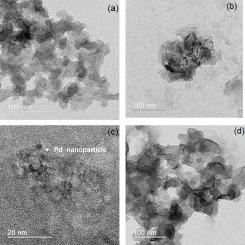
本研究首次描述了一种测定 2(3)-t-Butyl-4-hydroxyanisole (BHA) 的简单选择性方法,该方法基于在氢氧化铝基质中夹杂钯纳米颗粒(PdNPAH)的炭黑(CB)浆状电极。傅立叶变换红外光谱(FTIR)、扫描电子显微镜(SEM)和透射电子显微镜(TEM)对材料进行了表征。使用不同数量的每种材料对浆糊的成分进行了研究。对 BHA 在 PdNPAH/CBP 电极上的行为进行的伏安法研究表明,BHA 的氧化过程具有催化活性。伏安研究还表明,在 3,5- 二叔丁基-4-羟基甲苯(BHT)和 2-(1,1-二甲基乙基)-1,4-苯二酚(TBHQ)存在的情况下,可以测定 BHA。在优化条件下,BHA的线性范围为1-5000 µmol/L,检出限为0.20 µmol/L。该方法具有良好的选择性和精密度,并成功地应用于生物燃料中BHA的检测,回收率为98.80%-105.29%,表明该方法具有良好的准确性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploiting a carbon black paste electrode modified with palladium nanoparticles entrapped in aluminum hydroxide matrix for selective detection of 2(3)-t-Butyl-4-hydroxyanisole antioxidant in biodiesel samples
A simple and selective method for determination of 2(3)-t-Butyl-4-hydroxyanisole (BHA) based on carbon black (CB) paste electrode modified with Palladium nanoparticles entrapped in aluminum hydroxide matrix (PdNPAH) is described for the first time. The materials were characterized by Fourier-Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Transmission Electron Microscopy (TEM). The composition of the paste was studied using different amounts of each material. The voltammetric studies of the behavior of BHA on PdNPAH/CBP electrode demonstrated a catalytic activity for the oxidation process of BHA. Also, the voltammetric studies showed that it is possible to determine BHA in presence of 3,5-Di-tert-butyl-4-hydroxytoluene (BHT) and 2-(1,1-Dimethylethyl)-1,4-benzenediol (TBHQ). Under optimized conditions, the method showed a linear response for BHA from 1-5000 µmol/L and a detection limit of 0.20 µmol/L. The proposed method presented good selectivity, precision and it was successfully applied for detection of BHA in biofuel with recovery values from 98.80 % to 105.29 %, showing a good accuracy for the proposed method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


