探索煤热解芳香模型化合物在 Ca(OH)2 上的催化转化
IF 5.6
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
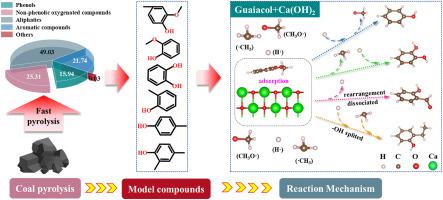
在分子水平上研究了 Ca(OH)2 催化煤中芳香模型化合物热解产物的分布。使用 Py-GC/MS 分析了煤热解产物的组成和相对丰度。煤在 600 °C 时的快速热解产物包括酚类(15.94 %)、非酚类含氧化合物(25.31 %)、脂肪族(49.03 %)、芳香族化合物(21.74 %)和其他化合物(0.03 %)。选择了六种具有代表性的芳香族模型化合物(2-甲氧基-4-甲基苯酚、对甲酚、2,4-二甲基苯酚、邻甲酚、愈创木酚和邻苯二酚)。模型化合物的热解过程主要是 C-O 和 C-C 键的裂解,从而形成甲氧基和甲基自由基。结果表明,Ca(OH)2 与 -OH 发生酸碱反应,从而提高了模型化合物的稳定性。值得注意的是,在同时含有 -OCH3 和 -OH 的芳香族化合物中,Ca(OH)2 对热解产物组成和分布的影响要比只含有 -OH 的化合物明显。通过密度泛函理论(DFT)计算,阐明了愈创木酚和 Ca(OH)2 热解产物的形成途径,证明 Ca(OH)2 可促进更多自由基的释放和模型化合物的转化。这项研究有助于从分子水平理解煤热解过程中芳香族化合物的转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the catalytic conversion of aromatic model compounds of coal pyrolysis over Ca(OH)2
The distribution of pyrolysis products from aromatic model compounds in coal catalyzed by Ca(OH)2 was investigated at the molecular level. The composition and relative abundance of the pyrolysis products from coal were analyzed using Py-GC/MS. The rapid pyrolysis products of coal at 600 °C consisted of phenols (15.94 %), non-phenolic oxygenated compounds (25.31 %), aliphatics (49.03 %), aromatic compounds (21.74 %), and other compounds (0.03 %). Six representative aromatic model compounds (2-methoxy-4-methylphenol, p-cresol, 2,4-dimethylphenol, o-cresol, guaiacol, and catechol) were selected. The pyrolysis process of model compounds was primarily the cleavage of C-O and C-C bonds, which resulted in the formation of methoxy and methyl radicals. The results revealed that Ca(OH)2 undergoes acid-base reactions with -OH, thereby increasing the stability of the model compounds. Notably, the impact of Ca(OH)2 on the composition and distribution of pyrolysis products was significantly more pronounced in aromatic compounds containing both -OCH3 and -OH compared to those containing solely -OH. The formation pathways of pyrolysis products involving guaiacol and Ca(OH)2 were elucidated through density functional theory (DFT) calculations, demonstrating that Ca(OH)2 could facilitate more free radicals release and the conversion of model compounds. This study contributes to the understanding of the transformation of aromatic compounds during coal pyrolysis at the molecular level.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


