在 SAPO-34 和 SAPO-5 的存在下,微波辅助从甘油和丙酮合成溶酮醛
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
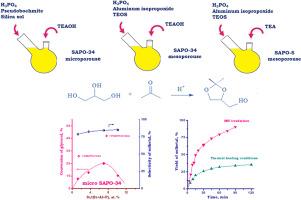
Solketal 是一种最有前途的添加剂,可改善汽车燃料的性能。有关从甘油和丙酮合成 Solketal 的强化工艺的研究越来越重要。本研究说明了在有硅铝磷酸盐(SAPO)作为催化剂的情况下,利用微波模式强化该工艺的情况。研究的重点是通过改变合成参数,如铝源和硅源的性质以及结构引导剂,来调整 SAPO-34 和 SAPO-5 的质地、酸性和催化特性。在微波辅助下,在丙酮/甘油摩尔比为 2.4 的甲醇溶液(甘油/甲醇:1 克/1 毫升)中,温度为 56 °C,研究了 SAPO 材料从甘油和丙酮合成溶酮醛的催化特性。结果表明,在所研究材料的存在下,对 Solketal 的选择性为 91.1-98.6%。与在氢氧化四乙胺存在下制备的微孔 SAPO-34 相比,在三乙胺存在下制备的介孔 SAPO-34 和 SAPO-5 具有更高的活性,这是因为它们具有较高的介孔率和更多的酸位点。在介孔 7.6%SAPO-34 存在下 90 分钟,观察到甘油的转化率为 90.4%,对索酮醛的选择性为 98.6%。使用微波技术的优势显而易见。在微波辐照下进行的反应中,索酮醛的产量比在热加热条件下进行的反应高出 3 倍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Microwave-assisted synthesis of solketal from glycerol and acetone in the presence of SAPO-34 and SAPO-5
Solketal is one of the most promising additives improving the properties of motor fuels. Investigations related to process intensification of synthesis of solketal from glycerol and acetone is gaining importance. The present work illustrates the use of the microwave mode for intensification of this process in the presence of silicoaluminophosphates (SAPO) as catalysts. The main focus of the study was placed on tuning the textural, acidic, and catalytic properties of SAPO-34 and SAPO-5 via variation of the synthesis parameters, such as the nature of Al and Si sources and the structure-directing agent. The catalytic properties of SAPO materials were investigated in the synthesis of solketal from glycerol and acetone under microwave assistance at the acetone/glycerol molar ratio of 2.4 in a methanol solution (glycerol/methanol: 1 g/1 mL) and 56 °C. It was demonstrated that the selectivity towards solketal was 91.1–98.6 % in the presence of the studied materials. The activities of mesoporous SAPO-34 and SAPO-5 prepared in the presence of triethylamine were higher as compared with microporous SAPO-34 prepared in the presence of tetraethylammonium hydroxide due to the high mesoporosity and a larger content of acid sites. The 90.4 % conversion of glycerol and 98.6 % selectivity towards solketal were observed in the presence of mesoporous 7.6%SAPO-34 for 90 min. The advantage of using microwave technology is shown. The solketal yield in the reaction under microwave irradiation was 3 times higher than that in the process performed under thermal heating conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


