氧化亚铜的表面化学
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
铜的化学和电子特性加上其巨大的天然丰度,使这种材料能够影响广泛的技术应用,包括异相催化。铜在 Cu1+ 氧化态时的反应活性使得这种特定构型与各种化学反应相关,但铜的易氧化还原特性使得分离单个态进行基础研究十分困难。在此,我们回顾了利用表面科学技术研究 Cu1+ 与小分子相互作用的三种 Cu2O 模型体系:Cu2O/Cu(111)、铜箔上的多晶 Cu2O 薄膜以及块状 Cu2O 晶体。通过对化学吸附和反应性研究的案例研究,讨论并举例说明了每种系统的优缺点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
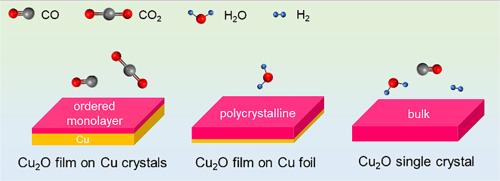
The surface chemistry of cuprous oxide
The chemical and electronic properties of copper combined with its large natural abundance lend this material to impact a wide range of technological applications, including heterogeneous catalysis. The reactivity of copper in its Cu1+oxidation state makes this specific configuration relevant in various chemical reactions, but the facile redox properties of copper make the isolation of individual states for fundamental studies difficult. Here we review three Cu2O model systems used to study the interaction of Cu1+ with small molecules making use of surface science techniques: Cu2O/Cu(111), thin polycrystalline Cu2O films on Cu foil, and bulk Cu2O crystals. Advantages and disadvantages of each system are discussed and exemplified through case studies of chemical adsorption and reactivity studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


