单晶氧化铁薄膜在电化学环境中的稳定性和溶解性
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过 X 射线光电子能谱、温度编程解吸、原位扫描隧道显微镜和循环伏安法,研究了铂(111)上单晶单层 FeO(111) 和 10 nm 薄 Fe3O4(111) 膜在化学复杂性不断增加的环境中的稳定性。在超高真空条件下制备的定义明确的氧化物薄膜暴露于不同 pH 值的水溶液中,并在纯电解液和含邻苯二酚的电解液中进行电化学循环。在中性(pH 值为 7)和碱性(pH 值为 13)溶液中,薄膜在开路条件下和电化学循环过程中在氢和氧进化电位范围内都很稳定。此外,在强酸性(pH 值为 1)高氯酸盐溶液中,薄膜在开路条件下保持完好,但在施加电化学势时会迅速溶解。特别是对于超薄的 FeO(111) 薄膜,在电化学循环过程中,邻苯二酚会增强薄膜在中性 pH 值下的溶解。通过比较 Pt(111)、FeO(111)和 Fe3O4(111)基底在电化学儿茶酚氧化反应中的作用,发现 FeO 在碱性环境中的活性增强且持续,而 Pt(111) 和 Fe3O4(111) 则出现强烈的失活现象。这是因为与其他基底相比,邻苯二酚与 FeO(111) 之间的相互作用较弱,阻碍了电极表面阻挡层的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
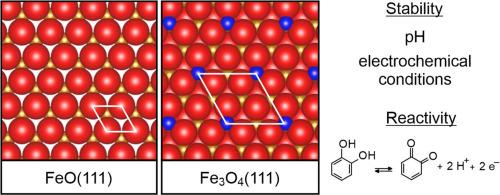
Stability and dissolution of single-crystalline iron oxide thin films in electrochemical environments
The stability of single-crystalline monolayer FeO(111) and 10 nm thin Fe3O4(111) films on Pt(111) upon exposure to environments of increasing chemical complexity has been studied with X-ray photoelectron spectroscopy, temperature-programmed desorption, in-situ scanning tunneling microscopy, and cyclic voltammetry. The well-defined oxide films, which were prepared under ultrahigh-vacuum conditions, were exposed to aqueous solutions of different pH and electrochemical cycling in pure and catechol-containing electrolyte. The films are stable in neutral (pH 7) and alkaline (pH 13) solutions both at open circuit conditions and during electrochemical cycling within the limits of hydrogen and oxygen evolution potentials. Also in strongly acidic (pH 1) perchlorate solution the films remain intact under open circuit conditions, but they quickly dissolve on application of electrochemical potential. Especially for the ultrathin FeO(111) films, catechol enhances the dissolution at neutral pH during electrochemical cycling. A comparison of Pt(111), FeO(111) and Fe3O4(111) substrates in the electrochemical catechol oxidation reaction reveals enhanced and sustained activity of FeO in alkaline environment, while strong deactivation occurs on Pt(111) and Fe3O4(111). This is explained by the weaker interaction between catechol and FeO(111) compared to the other substrates, which hampers the formation of a barrier layer on the electrode surface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


