利用 g-C3N4/GO/La2O3 光催化剂在可见光诱导下对农业水产废弃物中的毒死蜱进行连续光降解的过程
IF 3.8
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
杀虫剂的广泛使用以及这些杀虫剂在逐渐分解过程中形成的副产品导致了环境污染,进而对人类和生态系统的健康造成危害。世界各地的水体中都发现了杀虫剂,令人担忧。过去几十年来,光催化反应在分解杀虫剂方面受到了极大关注。研究了不同的参数,包括 pH 值、动力学、剂量和再生的影响。紫外可见光谱结果表明,与纯 g-C3N4 和 GO/ g-C3N4 相比,g-C3N4/GO/La2O3 纳米复合材料在降解毒死蜱(CPF)方面是一种更优越的可重复使用的光催化剂。g-C3N4/GO/La2O3 纳米复合材料的性能优于这两种材料,就证明了这一点。光催化性能的提高可能归因于带隙、形态、结晶质量和表面积之间的平衡,所有这些因素都可能减缓电子-空穴重组速率。这可能是光催化性能增强的原因。此外,还通过自由基淬灭研究概述了可行的过程,结果清楚地表明,在使用新型 g-C3N4/GO/La2O3 纳米复合材料进行高效光降解的过程中,更多 OH 自由基的存在起着至关重要的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
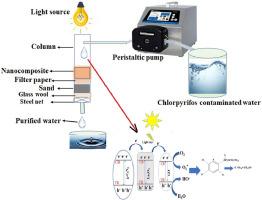
Visible light-induced continuous process for photodegradation of chlorpyrifos using g-C3N4/GO/La2O3 photocatalyst from agricultural aquatic waste
The widespread use of pesticides and the formation of by-products on the gradual decomposition of these pesticides have led to environmental pollution, which in turn has caused harm to both human and ecosystem health. Pesticides have been found in water bodies worldwide and are a cause of concern. Photocatalytic reactions have received significant attention in the past few decades for the breakdown of pesticides. Different parameters were studied, including the effects of pH, kinetics, dose, and regeneration. The UV–vis spectroscopy results suggest that the g-C3N4/GO/La2O3 nanocomposite is a superior reusable photocatalyst for the degradation of chlorpyrifos (CPF) compared to pure g-C3N4 and GO/ g-C3N4. This is demonstrated by the fact that the g-C3N4/GO/La2O3 nanocomposite outperforms both of these materials. The increased photocatalytic performance may be attributed to a balance between the band gap, morphology, crystalline quality, and surface area, all of which may be slowing down the electron-hole recombination rates. This may be due to the enhanced photocatalytic performance. In addition, the feasible processes were outlined from radical quenching studies, and the results clearly indicate that the presence of more OH radicals plays an essential role in the process of efficient photodegradation using novel g-C3N4/GO/La2O3 nanocomposites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


