立方 L12 HfAl3-xZnx 相的相稳定性实验测定
IF 3.1
2区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
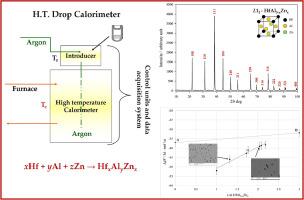
本研究致力于对立方体 L12-HfAl3-xZnx 固溶体的均匀性范围和形成热(ΔfH°,300 K)进行实验研究。我们使用高温直接滴热量计合成并同时测定了 HfZn3-HfAl3 部分(25% Hf)几种合金的 ΔfH°,同时使用 X 射线粉末衍射 (XRPD) 和扫描电子显微镜 (SEM) 搭配 EDS(能量色散光谱仪检测器)对样品进行表征。所进行的分析证实,三元 HfAl3-xZnx 合金在 1 ≤ x ≤ 2.24 的范围内几乎是单相的,具有立方 L12 结构;这反过来又有助于确定 L12 晶格参数(室温下)随成分变化的趋势。通过对我们的实验数据进行插值,确定了 L12-HfAl3-xZnx 的 ΔfH° 值(300 K 时为 kJ/mol-原子):-37.1 ± 2.0(HfAl0.8Zn2.2 对应 Hf25Al20Zn55 at.%)、-41.7 ± 2.0(HfAl1.2Zn1.8,相当于 Hf25Al30.0Zn45.0 at.%)、-45.1 ± 2.0(HfAl1.5Zn1.5,相当于 Hf25Al37.5Zn37.5 at.%)和 -48.5 ± 2.0(HfAl1.8Zn1.2,相当于 Hf25Al45.0Zn30.0 at.%)。对于两种相关的二元金属间相,在 300 K 时获得了以下 ΔfH° 值(单位:kJ/mol-atom):HfZn3(未知结构)为 -31.8 ± 3.0,HfAl3(四方 DO23 型结构)为 -37.0 ± 2.0。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Experimental determination of phase stability of the cubic L12 HfAl3-xZnx phase
The present study is devoted to the experimental investigation of homogeneity range and heat of formation (ΔfH° at 300 K) of the cubic L12–HfAl3-xZnx solid solution. A high-temperature direct drop calorimeter has been employed to synthesize and simultaneously determine the ΔfH° of several alloys along the HfZn3–HfAl3 section (25 at. % Hf) whereas X-Ray Powder Diffraction (XRPD) and Scanning Electron Microscopy (SEM) paired with an EDS (Energy Dispersive Spectrometer detector) have been employed to characterize the samples. The performed analysis confirmed that the ternary HfAl3-xZnx alloys were nearly single phase in the range 1 ≤ x ≤ 2.24 having the cubic L12 structure; this in turn helps establish the trend of L12 lattice parameter (at room temperature) with composition. Thanks to the interpolation of our experimental data, the following values of ΔfH° (kJ/mol-atom at 300 K) for the L12–HfAl3-xZnx were determined: -37.1 ± 2.0 (HfAl0.8Zn2.2 corresponding to Hf25Al20Zn55 at. %), -41.7 ± 2.0 (HfAl1.2Zn1.8 corresponding to Hf25Al30.0Zn45.0 at. %), -45.1 ± 2.0 (HfAl1.5Zn1.5 corresponding to Hf25Al37.5Zn37.5 at. %) and -48.5 ± 2.0 (HfAl1.8Zn1.2 corresponding to Hf25Al45.0Zn30.0 at. %). For two pertinent binary intermetallic phases, the following ΔfH° values (in kJ/mol-atom) at 300 K have been obtained: -31.8 ± 3.0 for HfZn3 (unknown structure) and -37.0 ± 2.0 for HfAl3 (tetragonal DO23 – type structure).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Thermochimica Acta
化学-分析化学
CiteScore
6.50
自引率
8.60%
发文量
210
审稿时长
40 days
期刊介绍:
Thermochimica Acta publishes original research contributions covering all aspects of thermoanalytical and calorimetric methods and their application to experimental chemistry, physics, biology and engineering. The journal aims to span the whole range from fundamental research to practical application.
The journal focuses on the research that advances physical and analytical science of thermal phenomena. Therefore, the manuscripts are expected to provide important insights into the thermal phenomena studied or to propose significant improvements of analytical or computational techniques employed in thermal studies. Manuscripts that report the results of routine thermal measurements are not suitable for publication in Thermochimica Acta.
The journal particularly welcomes papers from newly emerging areas as well as from the traditional strength areas:
- New and improved instrumentation and methods
- Thermal properties and behavior of materials
- Kinetics of thermally stimulated processes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


