通过非等温差示扫描量热法研究乙酰丙酮锆(IV)引发的 L-内酰胺大体积聚合动力学
IF 3.1
2区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
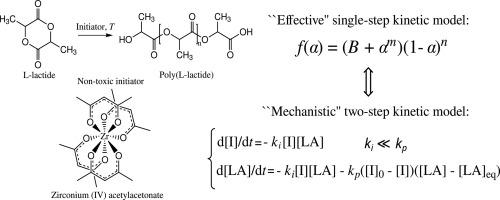
采用非等温差示扫描量热法研究了无毒引发剂乙酰丙酮锆(IV)引发的 L-内酯批量聚合的动力学。聚合动力学分析采用了等转换 "无模型 "法和模型拟合法相结合的方法。结果表明,无模型分析得出了一个自催化反应模型函数。为了给这一有效的反应模型提供物理意义,采用了考虑到不可逆启动和可逆传播反应的两步动力学模型进行模型拟合分析。结果表明,即使是这种简单的多步动力学模型也能解释等转化分析所揭示的聚合反应的一般特征,即有效活化能随转化率的变化和有效反应模型的自催化特性。对引发反应的速率常数 ki 和传播反应的速率常数 kp 进行了评估。结果表明,ki 的值比 kp 的值低约小数点后两位,这表明在所研究的温度范围内,起始速度较慢。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kinetics of L-lactide bulk polymerization initiated with zirconium(IV) acetylacetonate studied by non-isothermal differential scanning calorimetry
The kinetics of L-lactide bulk polymerization initiated with a non-toxic initiator, zirconium(IV) acetylacetonate, was studied by non-isothermal differential scanning calorimetry. The polymerization kinetics was analyzed using a combination of isoconversional “model–free” and model–fitting methods. It was revealed that the model–free analysis results in an autocatalytic reaction model function. To provide a physical meaning for this effective reaction model, a model–fitting analysis with a two–step kinetic model taking into account irreversible initiation and reversible propagation reactions was applied. It was demonstrated, that even such simple multi–step kinetic model can explain the general features of the polymerization reaction revealed by the isoconversional analysis, i.e. a variation of the effective activation energy with a conversion degree and an autocatalytic character of the effective reaction model. The rate constants for the initiation, , and propagation, , reactions were evaluated. It was revealed that the values of are about two decimal orders lower than that of indicating slow initiation in the studied temperature range.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Thermochimica Acta
化学-分析化学
CiteScore
6.50
自引率
8.60%
发文量
210
审稿时长
40 days
期刊介绍:
Thermochimica Acta publishes original research contributions covering all aspects of thermoanalytical and calorimetric methods and their application to experimental chemistry, physics, biology and engineering. The journal aims to span the whole range from fundamental research to practical application.
The journal focuses on the research that advances physical and analytical science of thermal phenomena. Therefore, the manuscripts are expected to provide important insights into the thermal phenomena studied or to propose significant improvements of analytical or computational techniques employed in thermal studies. Manuscripts that report the results of routine thermal measurements are not suitable for publication in Thermochimica Acta.
The journal particularly welcomes papers from newly emerging areas as well as from the traditional strength areas:
- New and improved instrumentation and methods
- Thermal properties and behavior of materials
- Kinetics of thermally stimulated processes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


