增强 ZnO@BiOX(X=Cl、Br、I)异质结在可见光下光催化降解新兴污染物的协同性能
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-09-25
DOI:10.1016/j.jphotochem.2024.116035
引用次数: 0
摘要
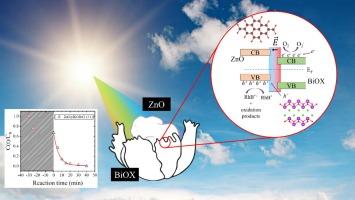
在这项工作中,我们研究了氧化锌和氧卤化铋异质结 ZnO@BiOX(其中 X=Cl-、Br-、I- 和它们的几种二元组合)中存在的卤素比例和卤素类型对在可见光下降解罗丹明-B(Rh-B)以及两种重要的新兴污染物间苯二酚和磺胺嘧啶的光催化活性的影响。这些材料是以 ZnO 纳米粒子和 BiOX 前体(Bi+ 和 X=Cl-、Br-、I- 离子)为起点,在 130 ℃ 下通过溶解热法合成的。研究发现,在降解 Rh-B(C0 = 30 ppm,VRxn = 250 mL)时,卤素的最佳组合为 75 % Br- 和 25 % Cl-(ZnO@BiOBrCl 溴氯比 3:1),形成的 Z 型异质结的时间常数为 τ = 5.6067 分钟(κ = τ-1 = 0.1784 分钟-1)。同样的复合材料在 250 分钟内完全降解了间苯二酚,在 150 分钟内降解了 85.2% 的磺胺嘧啶。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced Synergistic performance of ZnO@BiOX (X=Cl, Br, I) heterojunction for photocatalytic degradation of emerging pollutants under visible light
In this work, we study the influence of the proportion and halogen type present in heterojunctions of zinc oxide and bismuth oxyhalides ZnO@BiOX (Where X=Cl-, Br-, I- and several binary combinations of them) on the photocatalytic activity in the degradation of rhodamine-B (Rh-B) under visible light, as well as in two important emergent contaminants resorcinol and sulfadiazine. The materials were synthesized by a solvothermal process at 130 °C, starting from ZnO nanoparticles and BiOX precursors (Bi+ and X=Cl-, Br-, I- ions). It was found that the best combination of halogens was 75 % Br- and 25 % Cl- (ZnO@BiOBrCl bromine-chlorine ratio 3:1) forming a Z-type heterojunction with a time constant of τ = 5.6067 min (κ = τ-1 = 0.1784 min−1) in the degradation of Rh-B (C0 = 30 ppm, VRxn = 250 mL). The same composite degraded totally resorcinol in 250 min, and 85.2 % of sulfadiazine in 150 min.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


