酸性剥落氮化石墨(g-C3N4)在阳光直射下降解染料的光催化性能
IF 5.9
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氮化石墨碳(g-C3N4)是光催化应用中最有前途的半导体材料之一。然而,块状 g-C3N4 的光催化性能并不令人满意,原因在于其对可见光的吸收能力较差、重组速度快以及活性界面反应位点较少。在本研究中,我们通过酸(硝酸、盐酸和硫酸)剥离对块状 g-C3N4 进行了改性,以增强其对亚甲蓝(MB)和甲基橙(MO)染料的光催化降解能力。与原始 g-C3N4 相比,硫酸处理过的 g-C3N4 光催化剂(CN-S)对 MO 和 MB 的光催化降解效果显著。在阳光直射下 150 分钟,CN-S 对 MO 的光催化降解率为 96.89%,对 MB 的光催化降解率为 93.12%。自由基清除试验表明,与羟自由基(-OH)和光诱导空穴(h+)相比,超氧自由基(-O2-)对染料的光降解起主要作用。光致发光(PL)和时间分辨 PL 发射光谱表明,电子-空穴对(e-/h+)重组率低,电荷-载流子寿命长。此外,CN-S 在长达 5 次的运行中表现出优异的可回收性,但降解性能略有下降,MO 和 MB 染料的降解性能分别从 96.89% 和 93.12% 下降到 90.55% 和 88.84%。最终,研究结果表明,CN-S 是消除和降解废水中 MB 和 MO 染料的极佳光催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
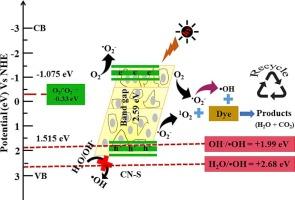
Photocatalytic performance of acid exfoliated graphitic carbon nitride (g-C3N4) for the degradation of dye under direct sunlight
Graphitic carbon nitride (g-C3N4) is one of the most promising semiconductor materials applied in photocatalytic applications. However, the photocatalytic performance of bulk g-C3N4 was not satisfactory due to poor visible-light absorption, quick recombination, and low amount of active interfacial reaction sites. In this study, we have modified the bulk g-C3N4 by acid (nitric, hydrochloric and sulphuric) exfoliation to enhance the photocatalytic degradation of methylene blue (MB) and methyl orange (MO) dye. Sulfuric acid-treated g-C3N4 photocatalyst (CN-S) presented significant photocatalytic degradation toward both MO and MB compared to the pristine g-C3N4. The photocatalytic degradation performance for CN-S is found to be ∼ 96.89 % for MO and ∼ 93.12 % for MB under 150 min under direct sunlight irradiation. Free radical scavenging tests showed the superoxide radicals (•O2−) were mostly responsible to the photodegradation of dyes while comparing to hydroxyl radicals (•OH) and photo-induced holes (h+). Which is attributed by Photoluminescence (PL) and time resolved PL emission spectra indicated a low electron-hole pair’s (e−/h+) recombination and longer charge-carrier lifetime. Moreover, the CN-S showed excellent recyclability for up to 5 runs with a slight reduction of degradation performance from 96.89 to 90.55 % for MO and 93.12 % to 88.84 % for MB dye, respectively. Ultimately, the results demonstrated that CN-S was a superb photocatalyst for the elimination and deterioration of MB and MO dyes from wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

FlatChem
Multiple-
CiteScore
8.40
自引率
6.50%
发文量
104
审稿时长
26 days
期刊介绍:
FlatChem - Chemistry of Flat Materials, a new voice in the community, publishes original and significant, cutting-edge research related to the chemistry of graphene and related 2D & layered materials. The overall aim of the journal is to combine the chemistry and applications of these materials, where the submission of communications, full papers, and concepts should contain chemistry in a materials context, which can be both experimental and/or theoretical. In addition to original research articles, FlatChem also offers reviews, minireviews, highlights and perspectives on the future of this research area with the scientific leaders in fields related to Flat Materials. Topics of interest include, but are not limited to, the following: -Design, synthesis, applications and investigation of graphene, graphene related materials and other 2D & layered materials (for example Silicene, Germanene, Phosphorene, MXenes, Boron nitride, Transition metal dichalcogenides) -Characterization of these materials using all forms of spectroscopy and microscopy techniques -Chemical modification or functionalization and dispersion of these materials, as well as interactions with other materials -Exploring the surface chemistry of these materials for applications in: Sensors or detectors in electrochemical/Lab on a Chip devices, Composite materials, Membranes, Environment technology, Catalysis for energy storage and conversion (for example fuel cells, supercapacitors, batteries, hydrogen storage), Biomedical technology (drug delivery, biosensing, bioimaging)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


