戊烯酸盐功能化透明质酸和戊烯酸盐功能化明胶水凝胶的表征,用于再生医学应用中的打印和未来手术置入
IF 3
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
与预制支架相比,原位交联的可注射水凝胶可能更适合用于临床损伤的外科手术。然而,如果低粘度水凝胶前体渗漏或被冲走,则可能难以通过手术将其植入损伤处。用于挤压生物打印的生物材料领域是发现具有可注射和糊状前体流变性及原位凝胶化能力的生物材料的沃土,这可能会促进材料在临床损伤中更好地保留。我们之前开发并评估了一种戊烯酸盐功能化透明质酸(PHA)/戊烯酸盐功能化明胶(PGel)水凝胶配方,这种水凝胶具有糊状、可印刷的前体,并能在脊髓损伤应用中快速光交联。为了扩大 PHA/PGel 水凝胶的生物打印和其他再生医学应用,需要进一步确定材料的特性以及细胞对 PHA/PGel 水凝胶配方的反应。在本研究中,我们利用二维核磁共振方法(即 1H-1H TOCSY)确认并量化了 PGel 和 PHA 的高度戊烯酸酯官能化。我们使用不同配方的 PGel 或 PHA/PGel 水凝胶对其硬度、膨胀性和细胞活力进行了表征。在压缩测试中,直接应用奥格登模型可以评估整个应力-应变范围,从而改进模量比较。我们确定了两种最能支持细胞存活的配方(即 3%/10% 和 4%/5% PHA/PGel)。此外,与另一种配方相比,其中一种配方(4%/5% PHA/PGel)具有更好的可印刷性。新的 PHA/PGel 水凝胶配方具有更好的可印刷性和潜在的临床手术植入性,可更广泛地应用于生物打印和再生医学领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
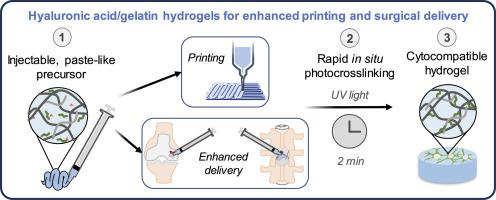
Characterization of pentenoate-functionalized hyaluronic acid and pentenoate-functionalized gelatin hydrogels for printing and future surgical placement in regenerative medicine applications
Injectable hydrogels with in situ crosslinking may be more suitable than pre-fabricated scaffolds for surgical delivery to clinical injuries. However, low viscosity hydrogel precursors may be challenging to surgically place into an injury if the precursor leaks or is washed out. The biomaterials field for extrusion bioprinting is a fertile ground for discovering biomaterials with injectable and paste-like precursor rheology with in situ gelation capabilities, which may promote better material retention in clinical injuries. We previously developed and evaluated one formulation of a pentenoate-functionalized hyaluronic acid (PHA) / pentenoate-functionalized gelatin (PGel) hydrogel with a paste-like, printable precursor and rapid photocrosslinking in a spinal cord injury application. Further characterization of the material and cell response to PHA/PGel hydrogel formulations is needed to expand the bioprinting and other regenerative medicine opportunities for PHA/PGel hydrogels. In the current study, we utilized 2D NMR methods (i.e., 1H–1H TOCSY) to confirm and quantify a high degree of pentenoate functionalization of PGel and PHA. We characterized the stiffness, swelling, and cell viability using varying formulations of PGel or PHA/PGel hydrogels. For compression testing, a straightforward application of the Ogden model enabled evaluation of the full stress-strain range for improved moduli comparisons. We identified two formulations that best supported cell viability (i.e., 3%/10% and 4%/5% PHA/PGel). Furthermore, one of the identified formulations (4%/5% PHA/PGel) had superior printability compared to the other. With better printability and potentially better clinical surgical placement, the new PHA/PGel hydrogel formulations may be more widely applied in the bioprinting and regenerative medicine fields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materialia
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
6.40
自引率
2.90%
发文量
345
审稿时长
36 days
期刊介绍:
Materialia is a multidisciplinary journal of materials science and engineering that publishes original peer-reviewed research articles. Articles in Materialia advance the understanding of the relationship between processing, structure, property, and function of materials.
Materialia publishes full-length research articles, review articles, and letters (short communications). In addition to receiving direct submissions, Materialia also accepts transfers from Acta Materialia, Inc. partner journals. Materialia offers authors the choice to publish on an open access model (with author fee), or on a subscription model (with no author fee).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


