透视钒氧化还原液流电池负极的电催化剂和性能障碍
IF 8.9
2区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
摘要
钒氧化还原液流电池(VRFB)因其储能容量大、性能稳定而被广泛应用于储能系统。钒氧化还原液流电池的关键部件之一是为氧化还原偶提供反应场所,因此理想的电极应具有优异的导电性、电化学和化学稳定性、良好的反应动力学以及低廉的价格。由于石墨毡(GF)和碳纸(CP)等碳基材料具有良好的特性,因此被广泛用作 VRFB 电极。然而,这些电极对 VO2+/VO2+ 和 V2+/V3+ 氧化还原偶的电化学活性较差,原因是动力学缓慢和极化较高,从而限制了 VRFB 在高电流密度下的运行。具体而言,由于 V2+/V3+ 反应与氢进化反应(HER)的电位范围重叠,负极的性能受到了限制,从而进一步影响了性能。研究人员开发了不同的策略来改善 VRFB 电极对 V2+/V3+ 反应的性能。在此,简要回顾了 VRFB 在 V2+/V3+ 反应中容量损失的主要原因,包括不良副反应,如氢进化反应和碳材料降解。重点介绍了电化学动力学、机理以及碳电极表面官能团的作用。此外,还综述了抑制负极 HER 的一般现象、机制和方法。此外,还概述了提高 VRFB 负半电池性能的方法。最后,确定了改善电极材料在 V2+/V3+ 反应中的性能所面临的挑战,并提出了未来的研究方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
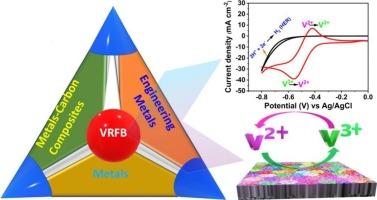
Perspective on electrocatalysts and performance hindrances at the negative electrode in vanadium redox flow batteries
Vanadium redox flow batteries (VRFBs) are widely used in energy storage systems due to their large storage capacity and stable performance. As one of the critical components of VRFBs to provide the reaction sites for redox couples, an ideal electrode should possess excellent conductivity, electrochemical and chemical stability, good reaction kinetics, and a low price. Due to their favorable properties, carbon-based materials such as graphite felt (GF) and carbon paper (CP) are widely used as VRFB electrodes. However, these electrodes suffer from poor electrochemical activity towards VO2+/VO2+ and V2+/V3+ redox couples, caused by sluggish kinetics and high polarization, limiting the operation of VRFB at high current density. Specifically, the negative electrode is performance-limiting due to the V2+/V3+ reaction overlapping with the potential range of the hydrogen evolution reaction (HER), further hindering performance. Researchers have developed different strategies to improve the performance of VRFB electrodes towards the V2+/V3+ reaction. Here, the leading causes of capacity losses in VRFB towards the V2+/V3+ reaction, including the undesirable side reactions, such as the HER and degradation of carbon materials, are briefly reviewed. The electrochemical kinetics, the mechanism, and the role of surface functional groups on carbon electrodes are highlighted. The general phenomena, mechanisms, and methods of HER inhibition in the negative electrode are also reviewed. Furthermore, approaches to improve the performance in the negative half-cell of VRFB are outlined. Finally, the ongoing challenges to improve the performance of electrode materials towards the V2+/V3+ reaction are identified, and future research directions are proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of energy storage
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
11.80
自引率
24.50%
发文量
2262
审稿时长
69 days
期刊介绍:
Journal of energy storage focusses on all aspects of energy storage, in particular systems integration, electric grid integration, modelling and analysis, novel energy storage technologies, sizing and management strategies, business models for operation of storage systems and energy storage developments worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


