具有光氧化诱导疗法的仿生聚合物纳米反应器,重振抗原依赖性和无抗原性免疫力
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
免疫细胞介导的抗癌模式通常会导致免疫细胞衰竭,对实体瘤的疗效有限。在此,我们开发了载氧生物仿生纳米反应器(BNR2(O2)),该反应器具有光氧化驱动疗法和抗原依赖性/无抗原性免疫重振功能,可对抗异种移植肿瘤。BNR2(O2)由聚合物纳米反应器组成,与癌细胞膜伪装在一起,可有效靶向同型肿瘤。与过氧化氢和谷胱甘肽孵育法相比,BNR2(O2)能持续释放氧气,促进细胞内活性氧(ROS)氧化二硒化键,从而可控地释放硒酸和抗叶酸培美曲塞。富含氧气的微环境可使培美曲塞增敏并阻断程序性细胞死亡配体 1(PD-L1),从而逆转 T 细胞免疫抑制。ROS 和培美曲塞可上调促凋亡蛋白并抑制叶酸相关酶,从而导致大量细胞凋亡和免疫原性细胞死亡,刺激树突状细胞成熟以改善细胞因子的分泌,扩大抗原依赖性 T 细胞免疫。此外,通过调节硒酸的释放,可以阻断肿瘤细胞的检查点受体人类白细胞抗原 E,从而重振无抗原的自然杀伤细胞免疫。这项研究将生物仿生纳米反应器与多种免疫细胞的调控结合起来,提供了一种先进的抗肿瘤策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
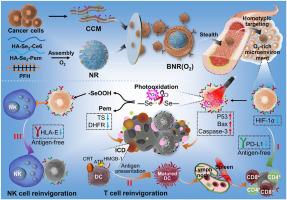
Biomimetic polymeric nanoreactors with photooxidation-initiated therapies and reinvigoration of antigen-dependent and antigen-free immunity
Immune cell-mediated anticancer modalities usually suffer from immune cell exhaustion and limited efficacy in solid tumors. Herein, the oxygen-carrying biomimetic nanoreactors (BNR2(O2)) have been developed with photooxidation-driven therapies and antigen-dependent/antigen-free immune reinvigoration against xenograft tumors. The BNR2(O2) composes polymeric nanoreactors camouflaged with cancer cell membranes can efficiently target homotypic tumors. It continuously releases O2 to boost intracellular reactive oxygen species (ROS) to oxide diselenide bonds, which controllably releases seleninic acids and anti-folate Pemetrexed compared to hydrogen peroxide and glutathione incubation. The O2-rich microenvironment sensitizes Pemetrexed and blocks programmed cell-death ligand 1 (PD-L1) to reverse T cell immunosuppression. The ROS and Pemetrexed upregulate pro-apoptosis proteins and inhibit folate-related enzymes, which cause significant apoptosis and immunogenic cell death to stimulate dendritic cell maturation for improved secretion of cytokines, expanding antigen-dependent T cell immunity. Furthermore, by regulating the release of seleninic acids, the checkpoint receptor human leukocyte antigen E of tumor cells can be blocked to reinvigorate antigen-free natural killer cell immunity. This work offers an advanced antitumor strategy by bridging biomimetic nanoreactors and modulation of multiple immune cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


