在 Ru-Na 协同界面上设计纳米粒子结构,实现二氧化碳捕获和氢化一体化
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
开发用于循环捕集和氢化二氧化碳的双功能材料对于将稀释的二氧化碳转化为有价值的燃料具有重要意义,但却受到动力学限制以及吸附剂和催化剂失活的困扰。在此,我们设计了一系列 RuNa/γ-Al2O3 材料,钌的大小从单个原子到团簇/纳米颗粒不等。我们在原子尺度上定量研究了钌的配位环境和结构敏感性。我们的研究结果表明,还原型 Ru 纳米粒子(直径约 7.1 nm,Ru-Ru 配位数为 5.9)在 340 °C 下具有很高的甲烷生成活性和选择性。Ru-Na 界面位点有助于二氧化碳通过脱氧途径迁移,包括碳酸盐解离、羰基形成和氢化。原位实验和理论计算表明,金属 Ru 纳米粒子上稳定的羰基中间体促进了异溶性 C-O 裂解和 C-H 键合,显著降低了激活储存的 CO2 的能量障碍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
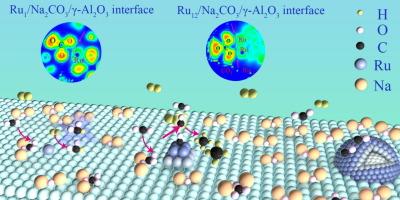
Engineering nanoparticle structure at synergistic Ru-Na interface for integrated CO2 capture and hydrogenation
The development of dual functional material for cyclic CO2 capture and hydrogenation is of great significance for converting diluted CO2 into valuable fuels, but suffers from kinetic limitation and deactivation of adsorbent and catalyst. Herein, we engineered a series of RuNa/γ-Al2O3 materials, varying the size of ruthenium from single atoms to clusters/nanoparticles. The coordination environment and structure sensitivity of ruthenium were quantitatively investigated at atomic scale. Our findings reveal that the reduced Ru nanoparticles, approximately 7.1 nm in diameter with a Ru-Ru coordination number of 5.9, exhibit high methane formation activity and selectivity at 340 °C. The Ru-Na interfacial sites facilitate CO2 migration through a deoxygenation pathway, involving carbonate dissociation, carbonyl formation, and hydrogenation. In-situ experiments and theoretical calculations show that stable carbonyl intermediates on metallic Ru nanoparticles facilitate heterolytic C–O scission and C–H bonding, significantly lowering the energy barrier for activating stored CO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


