采用硅-化学-体外(iSiCiV)综合方法确定生物标志物以预测植物化学物的皮肤致敏潜力
Q3 Pharmacology, Toxicology and Pharmaceutics
引用次数: 0
摘要
背景皮肤过敏是重要的监管终点之一,用于确定特定测试化学品是否会引起易感个体的过敏反应。预测化学品皮肤过敏可能性的体外方法已经发展成熟并被广泛接受。目的 用于皮肤治疗的草药制剂经常会引起皮肤过敏反应,因此需要谨慎对待。草药制剂往往含有一种以上的植物化学成分。预期的活性成分可能不会引起皮肤过敏。但是,其他植物化学物质可能会产生协同和增效作用,从而增强/抑制皮肤过敏。在这方面,分离、纯化、产量以及对所有痕量的皮肤过敏性进行体外/体内评估都将耗费大量时间、成本和动物。因此,监管机构希望使用计算工具来证实体外方法。监管机构还赞同使用定量结构-活性关系(QSAR)和读取交叉等室内方法。这些方法仅限于基于结构的预测,并与系统级方法相结合,可全面促进对复杂生物系统的更好理解。研究设计在本研究中,使用 OECD QSAR 工具箱对 603 种植物化学物质进行了室内筛选。方法开发了基于系统生物学的方法来补充基于 QSAR 的预测,并确定了 41 个生物标志物作为皮肤过敏的决定因素。结果有 31 种植物化学物质被预测为皮肤致敏物质。我们选择了丙烯醛、丁香酚、甲醛和甘油,使用直接肽反应性测定法 (DPRA)、KeratinoSens™ 和 h-CLAT 测定法对代表皮肤过敏过程中每个关键事件的内部预测进行了实验验证。此外,还利用 qRT-PCR 技术在 THP-1 和 KeratinoSens 细胞系中验证了已确定的生物标志物。在所有基因中,IL-8 和 CCL4 在 THP-1 中分别上调了 6.4 倍和 5.6 倍,而 AKR1C2 和 AKR1C3 在 KeratinoSens 中分别上调了 7.8 倍和 11.1 倍。 通过 ELISA 分析发现,IL-8 水平与 qRT-PCR 结果一致。该方法提供了一个稳健的平台,并强调了通过更好地了解毒理学机制来转变皮肤过敏化验模式的机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
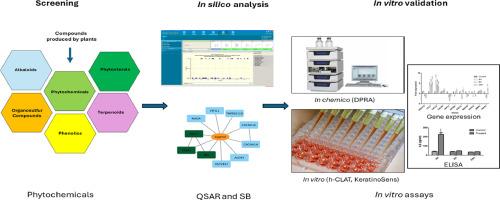
An integrated In-Silico-In-Chemico-In-Vitro (iSiCiV) Approach to identify biomarkers to predict the skin sensitisation potential of phytochemicals
Background
Skin sensitisation is one of the important regulatory endpoints used to determine whether a given test chemical can elicit an allergic response in susceptible individuals. The in-vitro approaches for predicting skin sensitisation potential for chemicals have been well-developed and widely accepted. Continuous efforts are being made to identify novel solutions to overcome the limitations of the existing approaches.
Purpose
The herbal preparations used in dermal therapeutics are frequently found to elicit skin sensitisation, requiring caution. The herbal preparations tend to contain more than one phytochemical. The expected active ingredient may not possess skin sensitisation. However, the other phytochemical traces could pose synergistic and potentiating effects towards enhanced/suppressed skin sensitisation. In this regard, isolation, purification, yield, and in-vitro / in-vivo evaluation of skin sensitisation for all the traces would be time, cost, and animal-consuming. Hence, the regulatory agencies appreciate using computational tools to substantiate in-vitro methods. The regulators have also endorsed using in-silico approaches like quantitative structure-activity relationships (QSAR) and read-across. These approaches are limited to structure-based predictions and in combination with system-level approaches, which comprehensively facilitate a better understanding of complex biological systems.
Study design
In the present study, 603 phytochemicals were screened in-silico using OECD QSAR Toolbox. The limitations of the QSAR Toolbox in predicting skin sensitisers were identified.
Method
The systems biology-based approach to complement the QSAR-based prediction was developed, and 41 biomarkers were identified as a determinant of skin sensitisation. The molecules attributed to these biomarkers were predicted as skin sensitisers.
Results
As a result, 31 phytochemicals were predicted as skin sensitisers. Acrolein, eugenol, formaldehyde, and glycerol were selected for experimental validation of the in-silico predictions using direct peptide reactivity assay (DPRA), KeratinoSens™, and h-CLAT assays representing each key event in skin sensitisation. Further, identified biomarkers were validated in THP-1 and KeratinoSens cell lines using qRT-PCR. Among all genes, IL-8 and CCL4 were upregulated by 6.4 and 5.6 folds, respectively, in THP-1, while AKR1C2 and AKR1C3 were upregulated by 7.8 and 11.1 folds, respectively, in KeratinoSens. IL-8 level was analysed by ELISA and found to be in line with qRT-PCR results.
Conclusion
The study proposes a possible strategy based on the “iSiCiV” approach to enhance the prediction of the dermal sensitisation potential of a test compound. The approach offers a robust platform and highlights an opportunity to shift the paradigm of skin sensitisation assay with a better understanding of toxicology mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine Plus
Medicine-Complementary and Alternative Medicine
CiteScore
3.70
自引率
0.00%
发文量
178
审稿时长
81 days
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


