是否应该使用十八种不相容药物?外用针叶和乌头治疗癌痛的镇痛效果的系统综述和荟萃分析
IF 1.7
4区 医学
Q3 INTEGRATIVE & COMPLEMENTARY MEDICINE
引用次数: 0
摘要
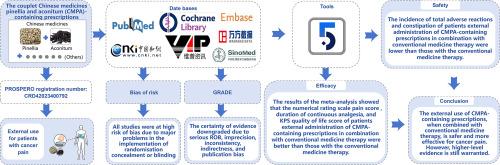
引言 含有中药松针和乌头(CMPA)的许多临床方剂已得到认可,并显示出镇痛疗效。然而,CMPA 属于中医理论中的 "十八反",被一些医家视为禁药和危险药。CMPA 能否真正安全无忧地应用于临床,迫切需要对证据进行更全面的评估。方法我们检索了PubMed、Embase、Cochrane Library、CNKI、万方、VIP和Sinomed数据库,收集了从开始到2023年12月31日期间比较CMPA与常规药物治疗癌痛的随机对照试验(RCT)。使用RevMan 5.4软件进行了荟萃分析。使用修改后的 Cochrane 偏倚风险工具评估纳入研究的质量,并构建了建议评估、发展和评价分级(GRADE)证据概况,以说明证据的确定性。荟萃分析结果显示,数字评分量表(NRS)疼痛评分[MD=-0.84,95 % CI (-1.21, -0.47),P <0.00001,中等确定性]、持续镇痛时间[MD=1.45 h,95 % CI (1.13, 1.77),P <0.00001,低确定性]和卡诺夫斯基表现状态(KPS)生活质量评分[MD=7.25,95 % CI (5.01,9.48),P < 0.00001,低确定性]。外用含 CMPA 处方联合常规药物治疗后,患者总不良反应[RR=0.69,95 % CI (0.58,0.83),P < 0.0001,中等确定性]和便秘[RR=0.43,95 % CI (0.19,0.97),P = 0.04,中等确定性]的发生率低于常规药物治疗。结论与传统药物疗法相比,外用含 CMPA 处方治疗癌痛更安全、更有效。综述注册PROSPERO注册号:CRD42023400792。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Should eighteen incompatibilities be used? A systematic review and meta-analysis of the analgesic effect of pinellia and aconitum used externally for cancer pain
Introduction
Many clinical formulas containing the couplet Chinese medicines pinellia and aconitum (CMPA) have been recognized and demonstrated analgesic efficacy. However, CMPA belongs to the "eighteen incompatibilities" of traditional Chinese medicine theory, which is considered prohibited and dangerous by some medical practitioners. Whether CMPA can truly be used safely and without concern in the clinic urgently requires a more cohesive evaluation of the evidence. This review aims to evaluate the effectiveness and safety of CMPA for external use in the treatment of cancer pain.
Methods
We searched PubMed, Embase, Cochrane Library, CNKI, Wanfang, VIP, and Sinomed databases to collect randomized controlled trials (RCTs) comparing CMPA with conventional medicines for the treatment of cancer pain from their inception to December 31, 2023. Meta-analysis was conducted using RevMan 5.4 software. The modified Cochrane risk of bias tool was used to assess the quality of the included studies, and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) evidence profile was constructed to illustrate the certainty of evidence.
Results
A total of 1083 patients were enrolled in 14 RCTs. The results of the meta-analysis showed that the numerical rating scale (NRS) pain score [MD=-0.84, 95 % CI (-1.21, -0.47), P < 0.00001, moderate certainty], duration of continuous analgesia [MD=1.45 h, 95 % CI (1.13, 1.77), P < 0.00001, low certainty], and Karnofsky performance status (KPS) quality of life score [MD=7.25, 95 % CI (5.01, 9.48), P < 0.00001, low certainty] of patients after external administration of CMPA-containing prescriptions in combination with conventional medicine therapy were better than those with the conventional medicine therapy. The incidence of total adverse reactions [RR=0.69, 95 % CI (0.58, 0.83), P < 0.0001, moderate certainty] and constipation [RR=0.43, 95 % CI (0.19, 0.97), P = 0.04, moderate certainty] of patients after external administration of CMPA-containing prescriptions in combination with conventional medicine therapy were lower than those with the conventional medicine therapy. No statistically significant differences were found regarding the effectiveness or safety of CMPA-containing prescriptions compared to conventional medicine therapy (low or very low certainty evidence).
Conclusion
The external use of CMPA-containing prescriptions, when combined with conventional medicine therapy, is safer and more effective for cancer pain than conventional medicine therapy. However, higher-level evidence is still warranted.
Review registration
PROSPERO registration number: CRD42023400792.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

European Journal of Integrative Medicine
INTEGRATIVE & COMPLEMENTARY MEDICINE-
CiteScore
4.70
自引率
4.00%
发文量
102
审稿时长
33 days
期刊介绍:
The European Journal of Integrative Medicine (EuJIM) considers manuscripts from a wide range of complementary and integrative health care disciplines, with a particular focus on whole systems approaches, public health, self management and traditional medical systems. The journal strives to connect conventional medicine and evidence based complementary medicine. We encourage submissions reporting research with relevance for integrative clinical practice and interprofessional education.
EuJIM aims to be of interest to both conventional and integrative audiences, including healthcare practitioners, researchers, health care organisations, educationalists, and all those who seek objective and critical information on integrative medicine. To achieve this aim EuJIM provides an innovative international and interdisciplinary platform linking researchers and clinicians.
The journal focuses primarily on original research articles including systematic reviews, randomized controlled trials, other clinical studies, qualitative, observational and epidemiological studies. In addition we welcome short reviews, opinion articles and contributions relating to health services and policy, health economics and psychology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


