通过在锂硫电池的 Ni2P 中引入掺硼原子和磷空位,促进硫的双向转化
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
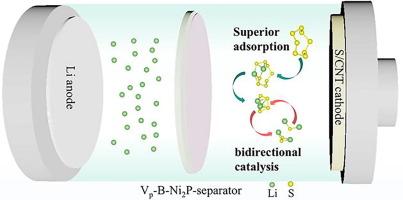
锂硫(Li-S)电池因其理论能量密度高、成本低而备受关注,但其进一步商业化却因臭名昭著的穿梭效应和缓慢的氧化还原动力学而受阻。在此,我们提供了一种优化 Ni2P 电子结构的策略,即在 Ni2P 中同时引入掺 B 原子和 P 空位(Vp-B-Ni2P),从而增强硫的双向转化。研究表明,在 Ni2P 中同时引入掺杂 B 原子和 P 空位会导致镍原子周围电子的重新分布,使镍原子的 d 带中心上移,镍原子与硫元素之间产生有效的 d-p 轨道杂化,从而加强了多硫化锂(LiPSs)的化学锚定,并加快了硫元素的双向转化动力学。同时,理论计算显示,Ni2P 中掺杂的 B 原子和 P 空位选择性地促进了 Li2S 的溶解和成核过程。因此,在 5 μL mg-1 的 E/S 和 7.20 mg cm-2 的硫载荷条件下,使用 Vp-B-Ni2P 隔离剂的锂-S 电池在 5 C 时具有 777 mA h g-1 的出色速率能力和 8.03 mA h cm-2 的高平均容量。这项工作阐明了在金属磷化物中引入杂原子和空位可协同调节电子结构,从而加速双向硫转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Boosting bidirectional sulfur conversion enabled by introducing boron-doped atoms and phosphorus vacancies in Ni2P for lithium-sulfur batteries
Lithium-sulfur (Li-S) batteries have gained great attention due to the high theoretical energy density and low cost, yet their further commercialization has been obstructed by the notorious shuttle effect and sluggish redox dynamics. Herein, we supply a strategy to optimize the electron structure of Ni2P by concurrently introducing B-doped atoms and P vacancies in Ni2P (Vp-B-Ni2P), thereby enhancing the bidirectional sulfur conversion. The study indicates that the simultaneous introduction of B-doped atoms and P vacancies in Ni2P causes the redistribution of electron around Ni atoms, bringing about the upward shift of d-band center of Ni atoms and effective d-p orbital hybridization between Ni atoms and sulfur species, thus strengthening the chemical anchoring for lithium polysulfides (LiPSs) as well as expediting the bidirectional conversion kinetics of sulfur species. Meanwhile, theoretical calculations reveal that the incorporation of B-doped atoms and P vacancies in Ni2P selectively promotes Li2S dissolution and nucleation processes. Thus, the Li-S batteries with Vp-B-Ni2P-separators present outstanding rate ability of 777 mA h g−1 at 5 C and high areal capacity of 8.03 mA h cm−2 under E/S of 5 μL mg−1 and sulfur loading of 7.20 mg cm−2. This work elucidates that introducing heteroatom and vacancy in metal phosphide collaboratively regulates the electron structure to accelerate bidirectional sulfur conversion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


