硬碳中的多硼掺杂效应可提高钠离子存储能力
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
硬碳(HC)一直被认为是钠离子电池(SIBs)的理想负极材料。优化硬碳的微观结构和固体电解质界面(SEI)特性已被证明能有效提高 Na+ 的存储能力,然而,一步到位的调节策略能同时实现多尺度的结构优化是非常可取的。在此,我们系统地研究了掺硼对硬碳微观结构和界面化学性质的影响。各种结构表征表明,适量的硼掺杂可以通过提高石墨化程度的碳层重排来增加封闭孔隙的尺寸,从而提供更多的 Na+ 储存位点。原位傅立叶变换红外光谱/电化学阻抗光谱(FTIR/EIS)和 X 射线光电子能谱(XPS)分析表明,BC3 结构增多,B-C-O 结构减少,从而增强了离子扩散动力学,形成了富含无机物的坚固 SEI,促进了电荷转移,实现了优异的速率性能。因此,优化了硼掺杂含量的硬碳阳极具有更高的速率和循环性能。总之,这项研究揭示了硼掺杂在优化硬碳的孔隙结构、界面化学和扩散动力学方面的关键作用,从而能够合理设计具有更强 Na+ 储存性能的钠离子电池阳极。本文章由计算机程序翻译,如有差异,请以英文原文为准。
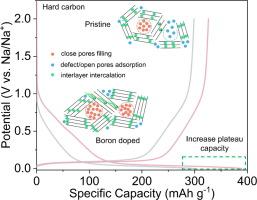
Multi boron-doping effects in hard carbon toward enhanced sodium ion storage
Hard carbon (HC) has been considered as promising anode material for sodium-ion batteries (SIBs). The optimization of hard carbon’s microstructure and solid electrolyte interface (SEI) property are demonstrated effective in enhancing the Na+ storage capability, however, a one-step regulation strategy to achieve simultaneous multi-scale structures optimization is highly desirable. Herein, we have systematically investigated the effects of boron doping on hard carbon’s microstructure and interface chemistry. A variety of structure characterizations show that appropriate amount of boron doping can increase the size of closed pores via rearrangement of carbon layers with improved graphitization degree, which provides more Na+ storage sites. In-situ Fourier transform infrared spectroscopy/electrochemical impedance spectroscopy (FTIR/EIS) and X-ray photoelectron spectroscopy (XPS) analysis demonstrate the presence of more BC3 and less B–C–O structures that result in enhanced ion diffusion kinetics and the formation of inorganic rich and robust SEI, which leads to facilitated charge transfer and excellent rate performance. As a result, the hard carbon anode with optimized boron doping content exhibits enhanced rate and cycling performance. In general, this work unravels the critical role of boron doping in optimizing the pore structure, interface chemistry and diffusion kinetics of hard carbon, which enables rational design of sodium-ion battery anode with enhanced Na+ storage performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


