等离子体串联异质结实现了高效电荷转移,可用于广谱光催化制氢
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
半导体光催化剂用于高效制氢的合理工程设计意义重大,但仍具有挑战性,这主要是由于电荷转移动力学的限制。本文旨在通过相结、肖特基结和光热效应的协同策略,制备一种迷人的 hc-CdS/Mo2C@C 异质结构的等离子体串联异质结,以有效促进 CdS 光催化剂的电荷分离动力学。立方CdS(c-CdS)和六方CdS(h-CdS)原子构型的差异通过典型的II电荷转移机制实现了有效的电荷分离,而等离子体肖特基结则进一步提取了hc-CdS相结中的电子,实现了梯度电荷转移。此外,Mo2C@C 的光热效应有助于扩大光吸收,加速电荷转移动力学,降低氢演化能垒。碳层为电荷转移提供了快速通道,并保护光催化剂免受光腐蚀。因此,优化后的 hc-CMC 光催化剂的光催化产氢活性高达 28.63 mmol/g/h,表观量子效率为 61.8%,超过了大多数已报道的光催化剂。这项研究为通过构建等离子体串联异质结来提高 CdS 的电荷转移动力学和光催化活性提供了一种可行的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
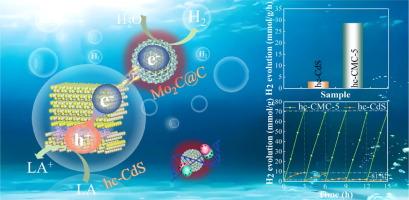
Plasmonic tandem heterojunctions enable high-efficiency charge transfer for broad spectrum photocatalytic hydrogen production
Rational engineering of semiconductor photocatalysts for efficient hydrogen production is of great significance but still challenging, primarily due to the limitations in charge transfer kinetics. Herein, a fascinating plasmonic tandem heterojunction with the hc-CdS/Mo2C@C heterostructure is aimfully prepared for effectively promoting the charge separation kinetics of the CdS photocatalyst via the synergistic strategy of phase junction, Schottky junction, and photothermal effect. The difference in atomic configuration between cubic-CdS (c-CdS) and hexagonal-CdS (h-CdS) leads to effective charge separation through a typical II charge transfer mechanism, and plasmonic Schottky junction further extracts the electrons in the hc-CdS phase junction to realize gradient charge transfer. Besides, the photothermal effect of Mo2C@C helps to expand the light absorption, accelerate charge transfer kinetics, and reduce the hydrogen evolution energy barrier. The carbon layer provides a fast channel for charge transfer and protects the photocatalyst from photocorrosion. As a result, the optimized hc-CMC photocatalyst exhibits a significantly high photocatalytic H2 production activity of 28.63 mmol/g/h and apparent quantum efficiency of 61.8%, surpassing most of the reported photocatalysts. This study provides a feasible strategy to enhance the charge transfer kinetics and photocatalytic activity of CdS by constructing plasmonic tandem heterogeneous junctions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


