介电离子导电 ZnNb2O6 层可实现无枝晶锌金属电池的快速脱溶和扩散
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
可充电水溶液锌金属电池(AZMB)具有成本低、安全性高等优点,是大规模储能系统的理想候选材料。然而,电极/电解质界面上迟缓的解溶胶动力学以及相应的氢进化反应(活性水分子紧密地参与到 Zn(H2O)62+ 溶胶壳中)极大地阻碍了它们的性能和可持续性。在此,我们从阳极中自生成的固体电解质相(SEI)中汲取灵感,在金属锌表面构建了介电但具有离子导电性的纳米铌酸锌人工层(ZNB@Zn),作为一种快速脱溶促进剂。电子/光学显微镜和界面光谱测量以及理论计算均证实,纳米铌酸锌层的亲锌和导电特性可加速界面脱溶/扩散,抑制表面腐蚀或枝晶的形成,实现均匀的镀锌/剥离行为。因此,制备的 ZNB@Zn 电极即使在高电流密度和深度放电条件下,也能表现出超过 2000 小时的优异循环稳定性和稳健的可逆性(99.54%)。同时,组装后的 ZNB@Zn 全电池在 5 A g-1 条件下循环 3000 次后显示出 80.21% 的高容量保持率和高达 10 A g-1 的出色速率性能。大实心小袋电池在数百次循环后仍能保持稳定,凸显了介电但离子导电层在 AZMB 进一步应用中的广阔前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
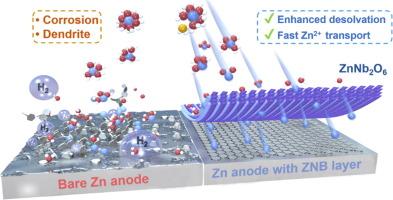
Dielectric-ion-conductive ZnNb2O6 layer enabling rapid desolvation and diffusion for dendrite-free Zn metal batteries
Rechargeable aqueous zinc-metal batteries (AZMBs) are promising candidates for large-scale energy storage systems due to their low cost and high safety. However, their performance and sustainability are significantly hindered by the sluggish desolvation kinetics at the electrode/electrolyte interface and the corresponding hydrogen evolution reaction where active water molecules tightly participate in the Zn(H2O)62+ solvation shell. Herein, learnt from self-generated solid electrolyte interphase (SEI) in anodes, the dielectric but ion-conductive zinc niobate nanoparticles artificial layer is constructed on metallic Zn surface (ZNB@Zn), acting as a rapid desolvation promotor. The zincophilic and dielectric-conductive properties of ZNB layer accelerate interfacial desolvation/diffusion and suppress surface corrosion or dendrite formation, achieving uniform Zn plating/stripping behavior, as confirmed by electronic/optical microscopies and interface spectroscopical measurements together with theoretical calculations. Consequently, the as-prepared ZNB@Zn electrode exhibits excellent cycling stability of over 2000 h and robust reversibility (99.54%) even under high current density and depth of discharge conditions. Meanwhile, the assembled ZNB@Zn-based full cell displays high capacity-retention rate of 80.21% after 3000 cycles at 5 A g−1 and outstanding rate performance up to 10 A g−1. The large-areal pouch cell is stabilized for hundreds of cycles, highlighting the bright prospects of the dielectric but ion-conductive layer in further application of AZMBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


