非晶钴金属硼化物上的痕量铜诱导低 C─N 耦合屏障,促进电化学尿素生产
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二氧化碳(CO2)和硝酸盐(NO3-)的电化学 C─N 偶联是尿素合成传统高能耗工业途径的替代策略,迫切需要设计高效催化剂以实现高产率和法拉第效率(FE)。本文设计了非晶态低含量铜掺杂钴茂金属硼化物(a-Cu0.1CoBx metallene),用于通过电化学 C─N 偶联合成尿素。在二氧化碳饱和的 0.1 m KNO3 电解质中,a-Cu0.1CoBx 茂金属能驱动 CO2 和 NO3- 的电催化 C─N 偶联合成尿素,在-0.5 V 时的 FE 为 27.7%,产率为 312 µg h-1 mg-1cat.,并且具有优异的循环稳定性。原位傅立叶变换红外光谱和理论计算显示,Cu、Co 和 B 之间的电子效应导致 Cu 和 Co 成为双活性位点,促进了反应物的吸附。此外,引入的痕量 Cu 降低了 C─N 偶联的反应能垒,从而促进了尿素的合成。这项工作为优化 Co 基金属烯通过 C─N 偶联电合成尿素提供了一条可行的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
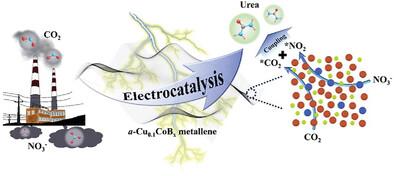
Trace Cu-Induced Low C─N Coupling Barrier on Amorphous Co Metallene Boride for Boosting Electrochemical Urea Production
The electrochemical C─N coupling of carbon dioxide (CO2) and nitrate(NO3-) is an alternative strategy to the traditional high−energy industrial pathway for urea synthesis, which urgently requires the design of efficient catalysts to achieve high yield and Faraday efficiency (FE). Here, amorphous low-content copper-doped cobalt metallene boride (a-Cu0.1CoBx metallene) is designed for urea synthesis via electrochemical C─N coupling. The a-Cu0.1CoBx metallene can drive electrocatalytic C─N coupling of CO2 and NO3− for urea synthesis in CO2-saturated 0.1 m KNO3 electrolyte, with 27.7% of FE and 312 µg h−1 mg−1cat. of yield at −0.5 V, as well as superior cycling stability. The in situ Fourier transform infrared and theoretical calculations reveal that electronic effect between Cu, Co, and B causes Cu and Co as dual active sites to promote the adsorption of reactants. Furthermore, the introduced trace Cu reduces the reaction energy barrier of the C─N coupling to facilitate urea synthesis. This work provides a promising route for the optimization of Co-based metallene for the electrosynthesis of urea through C─N coupling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


