烷基硼烷的铃木-宫浦交叉耦合:反金属化研究和有机钯预反金属化物种模型的合成
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本报告研究了 B-烷基-9-硼杂双环[3.3.1]壬烷(B-烷基-9-BBN)偶联剂在 Csp2-Csp3 Suzuki-Miyaura(SM)反应中的反金属化。我们合成了动力学上稳定的有机钯(II)SM 预金属化中间体模型,从而最终对这些难以捉摸的物种进行了全面的 X 射线晶体学和核磁共振光谱表征。对动力学相关体系的计算研究表明,通过前侧 SE2(配位)机制,在保留碳原子构型的情况下进行了反金属化。这涉及一个四元过渡态,电子转移到空置的钯 dx2-y2 轨道。此外,综合理论和实验结果表明,在氢氧化物存在的四氢呋喃/水混合物中,催化转化在动力学上更倾向于阴离子转金属路径 A,即[Pd(Ph)(PPh3)2Br]与[B-烷基-9-BBN(OH)]-发生初始反应,而不是中性路径 B,后者需要先形成[Pd(Ph)(PPh3)2OH],然后与 B-烷基-9-BBN 发生反应。值得注意的是,在路径 A 中,[Pd(Ph)(PPh3)2Br]只有在膦配体与[B-烷基-9-BBN(OH)]-发生交换后,溴的损失才会出现,才有可能转化为苯钯(II)硼酸盐预金属化物种。这些细微差别可能至少部分源于 B- 烷基-9-BBN 及其衍生物中硼中心极高的路易斯酸度和立体体积。本文章由计算机程序翻译,如有差异,请以英文原文为准。
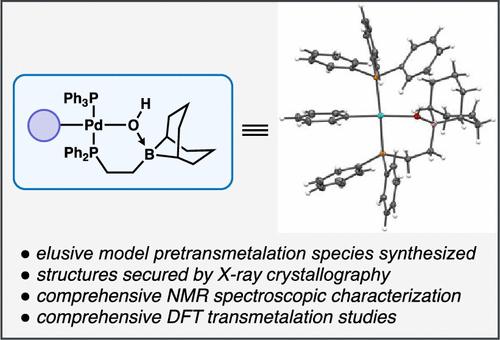
Suzuki–Miyaura Cross-Couplings of Alkylboranes: Transmetalation Studies and Synthesis of Model Organopalladium Pretransmetalation Species
This report investigates the transmetalation of B-alkyl-9-borabicyclo[3.3.1]nonane (B-alkyl-9-BBN) coupling partners in Csp2–Csp3 Suzuki–Miyaura (SM) reactions. Kinetically stable, model organopalladium(II) SM pretransmetalation intermediates were synthesized, which enabled comprehensive X-ray crystallographic and NMR spectroscopic characterization of these elusive species at last. Computational studies of kinetically relevant systems were consistent with transmetalation proceeding with retention of configuration with respect to the carbon atom via a frontside SE2 (coordination) mechanism. This is proposed to involve a four-membered transition state with electron transfer into the vacant palladium dx2–y2 orbital. Furthermore, integrated theoretical and experimental results indicated that in the presence of hydroxide in THF/water mixtures, catalytic turnover is kinetically favored via anionic transmetalation path A, which involves the initial reaction of [Pd(Ph)(PPh3)2Br] with [B-alkyl-9-BBN(OH)]−, over neutral path B, which requires initial formation of [Pd(Ph)(PPh3)2OH] then reaction with B-alkyl-9-BBN. It is notable that in path A, the conversion of [Pd(Ph)(PPh3)2Br] to the phenylpalladium(II) boronate pretransmetalation species only appears to be feasible if phosphine ligand exchange with [B-alkyl-9-BBN(OH)]− precedes the loss of bromide. It is likely that these nuances derive, at least partly, from the very high Lewis acidity and steric bulk of the boron center within B-alkyl-9-BBN and its derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


