光氧化催化炔溴化物与叔胺氮原子邻接的 C(sp3)-H 键的炔化反应
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在可见光辐照下,以炔基溴化物作为自由基炔化试剂,对邻近叔胺氮原子的 C(sp3)-H键进行了一种新颖而稳健的炔化反应。包括 N-芳基胺和 N-烷基胺在内的各种叔胺与芳香族和脂肪族溴化炔基化合物发生偶联反应,以中等到极好的收率(31-80% 的收率)制备出 51 种丙炔基胺。根据初步的机理研究,可能的机理是自由基加成-消除过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
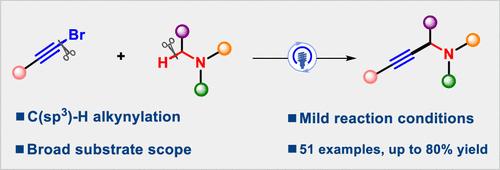
Photoredox-Catalyzed Alkynylation of C(sp3)-H Bonds Adjacent to a Nitrogen Atom of Tertiary Amines with Alkynyl Bromides
A novel and robust alkynylation of C(sp3)–H bonds adjacent to a nitrogen atom of tertiary amines with alkynyl bromides as radical alkynylating reagents has been realized under visible-light irradiation. A range variety of tertiary amines including N-arylamines and N-alkylamine have been coupled with both aromatic and aliphatic alkynyl bromides to furnish 51 examples of propargylamines in moderate to excellent yields (31–80% yields). The possible mechanism was a radical addition–elimination process based on preliminary mechanistic studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


