四氯化锆升华热力学
IF 0.4
Q4 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
摘要
研究了四氯化锆升华过程的热力学。研究表明,从伴生的五氯化铌中提纯 ZrCl4 原则上是可行的。四氯化锆中的部分铌可以三氯化铌氧的形式存在。三氯化铌在整个温度范围内几乎不挥发,并会浓缩在炭中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
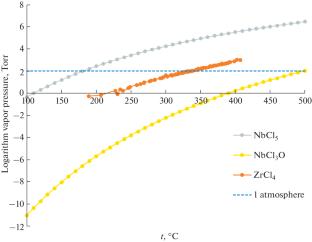
Thermodynamics of Sublimation Zirconium Tetrachloride
The thermodynamics of the sublimation process of zirconium tetrachloride has been studied. The possibility in principle to purify ZrCl4 from the accompanying niobium pentachloride is shown. Part of niobium in zirconium tetrachloride can be contained in the form of niobium oxytrichloride. Niobium oxytrichloride is virtually non-volatile over the entire temperature range and will concentrate in the char.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Metallurgy (Metally)
METALLURGY & METALLURGICAL ENGINEERING-
CiteScore
0.70
自引率
25.00%
发文量
140
期刊介绍:
Russian Metallurgy (Metally) publishes results of original experimental and theoretical research in the form of reviews and regular articles devoted to topical problems of metallurgy, physical metallurgy, and treatment of ferrous, nonferrous, rare, and other metals and alloys, intermetallic compounds, and metallic composite materials. The journal focuses on physicochemical properties of metallurgical materials (ores, slags, matters, and melts of metals and alloys); physicochemical processes (thermodynamics and kinetics of pyrometallurgical, hydrometallurgical, electrochemical, and other processes); theoretical metallurgy; metal forming; thermoplastic and thermochemical treatment; computation and experimental determination of phase diagrams and thermokinetic diagrams; mechanisms and kinetics of phase transitions in metallic materials; relations between the chemical composition, phase and structural states of materials and their physicochemical and service properties; interaction between metallic materials and external media; and effects of radiation on these materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


