改进硫酸浸出还原性红土铁矿参数并选择性回收镍和钴的可能性
IF 0.4
Q4 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
摘要
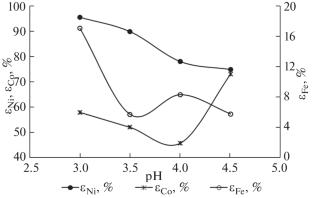
研究了通过弱硫酸溶液对还原亚铁氧化镍(褐铁矿)矿石进行湿法冶金处理的方案,从 Buruktal 矿床的亚铁红土矿石中回收镍和钴的情况。氨试剂 (NH4)2SO4 可形成镍和钴络合物,用于提高浸出选择性。研究了在提高溶液 pH 值(pH 值 3.0-5.0)和循环使用硫酸溶液的情况下,减少 H2SO4 消耗和提高镍和钴回收率的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Possibility of Improving Parameters of the Sulfuric Acid Leaching of Reduced Ferriferrous Laterite Ores with Selective Nickel and Cobalt Recovery
The recovery of nickel and cobalt from the ferriferrous laterite ores of the Buruktal deposit via a hydrometallurgical processing scheme for the reduced ferriferrous oxidized nickel (limonite) ore with weak sulfuric acid solutions is studied. An ammonia reagent (NH4)2SO4, which forms Ni and Co complexes, is used to enhance the leaching selectivity. The possibility of decreasing the consumption of H2SO4 and intensifying nickel and cobalt recovery at increased pH of the solution (pH 3.0–5.0) and recycling of sulfuric acid solutions is studied.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Metallurgy (Metally)
METALLURGY & METALLURGICAL ENGINEERING-
CiteScore
0.70
自引率
25.00%
发文量
140
期刊介绍:
Russian Metallurgy (Metally) publishes results of original experimental and theoretical research in the form of reviews and regular articles devoted to topical problems of metallurgy, physical metallurgy, and treatment of ferrous, nonferrous, rare, and other metals and alloys, intermetallic compounds, and metallic composite materials. The journal focuses on physicochemical properties of metallurgical materials (ores, slags, matters, and melts of metals and alloys); physicochemical processes (thermodynamics and kinetics of pyrometallurgical, hydrometallurgical, electrochemical, and other processes); theoretical metallurgy; metal forming; thermoplastic and thermochemical treatment; computation and experimental determination of phase diagrams and thermokinetic diagrams; mechanisms and kinetics of phase transitions in metallic materials; relations between the chemical composition, phase and structural states of materials and their physicochemical and service properties; interaction between metallic materials and external media; and effects of radiation on these materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


