淀粉样蛋白-β(1-40)寡聚体的临界聚集浓度和可逆性。
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
淀粉样蛋白-β(Aβ)聚集是阿尔茨海默病发病机制中的一个关键因素,其异构体淀粉样蛋白-β 1-40(Aβ40)和 1-42(Aβ42)的聚集行为截然不同。在这项研究中,我们利用荧光相关光谱(FCS)和详细的数据分析研究了 Aβ40 的聚集特性。我们的研究结果表明,Aβ40 经历了两步合作聚集过程。第一步的临界聚集浓度(cac)为 0.5 ± 0.3 μM,可形成 5 至 25 个单体的畸变低聚物和 50 至 100 个单体的稳定低聚物,聚集的淀粉样蛋白不到 10%。第二步的 cac 值为 18.9 ± 2.2 μM,形成的聚集体要大得多,与原纤维一致,聚集的淀粉样蛋白约占 50%。值得注意的是,与 Aβ42 相比,Aβ40 的 cac 值要高得多,而聚集的淀粉样蛋白的比例要低得多,这表明其聚集倾向较低。此外,我们的研究结果表明,Aβ40 早期低聚物在稀释后是可逆的,尽管存在分解的动力学障碍。这些对 Aβ40 聚集机制的深入研究加深了我们对其在阿尔茨海默病中作用的理解,并可能为针对淀粉样蛋白聚集的治疗策略提供依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
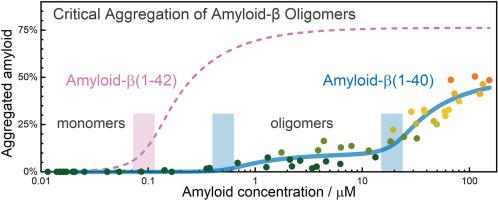
Critical aggregation concentration and reversibility of amyloid-β (1–40) oligomers
Amyloid-beta (Aβ) aggregation is a critical factor in the pathogenesis of Alzheimer's disease, with distinct aggregation behaviours observed between its isoforms Amyloid-β 1–40 (Aβ40) and 1–42 (Aβ42). In this study, we investigated the aggregation properties of Aβ40 using fluorescence correlation spectroscopy (FCS) and detailed data analysis. Our results reveal that Aβ40 undergoes a two-step cooperative aggregation process. The first step, characterized by a critical aggregation concentration (cac) of 0.5 ± 0.3 μM, results in the formation of metastable oligomers of 5–25 monomers and stable oligomers of 50–100 monomers, with less than 10 % of the total amyloid aggregated. The second step, with a cac of 19 ± 2 μM, leads to the formation of much larger aggregates, consistent with protofibrils, and approximately 50 % aggregated amyloid. Notably, the cac for Aβ40 is significantly higher, and the fraction of aggregated amyloid is much lower compared to Aβ42, indicating a lower propensity for aggregation. Additionally, our findings suggest that Aβ40 early oligomers are reversible upon dilution, albeit with a kinetic barrier to disaggregation. These insights into the aggregation mechanisms of Aβ40 enhance our understanding of its role in Alzheimer's disease and may inform therapeutic strategies targeting amyloid aggregation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


