(E)-Selective Weinreb Amide-Type Horner-Wadsworth-Emmons Reaction:反应条件的影响、底物范围、反应性膦酸镁的分离及应用。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-01
Epub Date: 2024-10-11
DOI:10.1021/acs.joc.4c01140
引用次数: 0
摘要
开发了一种 iPrMgCl 去质子化 Weinreb 酰胺型 Horner-Wadsworth-Emmons (HWE) 反应,并研究了碱、阳离子、溶剂和浓度等不同反应条件对扩大底物范围和实现高(E)选择性的影响。结果发现,与原位生成的膦酸烯酸盐相比,由 iPrMgCl 生成的 Weinreb 酰胺型膦酸烯酸盐可分离、稳定至少超过半年,并可用于 HWE 反应,保持较高的生产率和选择性。这些结果促使我们进行了一项应用研究,包括连续延伸、双环丙烷的合成和魏氏酮的合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
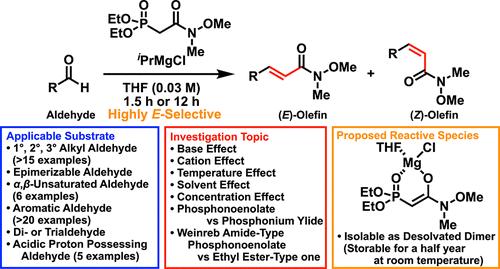
(E)-Selective Weinreb Amide-Type Horner-Wadsworth-Emmons Reaction: Effect of Reaction Conditions, Substrate Scope, Isolation of a Reactive Magnesium Phosphonoenolate, and Applications.
An iPrMgCl-deprotonating Weinreb amide-type Horner-Wadsworth-Emmons (HWE) reaction was developed, and the effects of diverse reaction conditions, including the base, cation, solvent, and concentration, were investigated to broaden the substrate scope and achieve high (E)-selectivity. The Weinreb amide-type phosphonoenolate generated from iPrMgCl was found to be isolable, stable for at least over a half year, and applicable in the HWE reaction keeping high productivity and selectivity compared with the in situ generated phosphonoenolate. The results prompted us to perform an application study including successive elongation, synthesis of a biscyclopropane, and Weinreb ketone syntheses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


